Volume Of A Bcc Unit Cell
Juapaving
Mar 20, 2025 · 5 min read
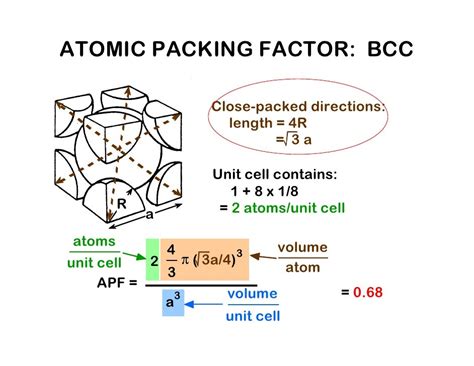
Table of Contents
Unveiling the Secrets of BCC Unit Cell Volume: A Comprehensive Guide
The world of crystallography is fascinating, filled with intricate structures and precise geometric relationships. Understanding these structures is crucial in various fields, from materials science and engineering to chemistry and physics. One fundamental concept in this realm is the unit cell, the smallest repeating unit of a crystal lattice. Among the various unit cell types, the body-centered cubic (BCC) unit cell holds a significant place, owing to its unique properties and prevalence in many metals and alloys. This comprehensive guide delves deep into the intricacies of calculating the volume of a BCC unit cell, explaining the underlying principles and providing practical examples to solidify your understanding.
Understanding the BCC Unit Cell Structure
Before we embark on calculating the volume, let's first establish a clear understanding of the BCC unit cell's structure. A BCC unit cell is characterized by its cubic shape, with lattice points located at each of the eight corners of the cube and one additional lattice point situated precisely at the center of the cube. This central atom is crucial in distinguishing a BCC structure from a simple cubic (SC) structure.
Key features of a BCC unit cell:
- Cubic Shape: The unit cell is a perfect cube.
- Eight Corner Atoms: Each corner of the cube contains a fraction of an atom.
- One Central Atom: A complete atom resides at the center of the cube.
- Lattice Points: The locations of the atoms within the unit cell define the lattice points.
- Atoms per Unit Cell: A BCC unit cell contains a total of two atoms (eight corner atoms, each contributing 1/8 of an atom, plus one central atom).
Calculating the Volume of a BCC Unit Cell: A Step-by-Step Guide
Calculating the volume of a BCC unit cell involves a straightforward approach once you understand the relationship between the unit cell edge length (a) and the atomic radius (r).
1. Defining the Relationship Between Edge Length (a) and Atomic Radius (r)
Imagine a body diagonal that cuts through the cube, connecting opposite corners. This diagonal passes through the center atom and two corner atoms. The length of this body diagonal can be expressed using the Pythagorean theorem in three dimensions.
Consider a right-angled triangle formed by:
- One edge of the cube (length a).
- The diagonal of one face of the cube (length √2a).
- The body diagonal (length 4r).
Using the Pythagorean theorem, we arrive at the relationship:
(4r)² = a² + (√2a)²
Simplifying this equation, we get:
16r² = 3a²
This crucial equation allows us to express the edge length (a) in terms of the atomic radius (r):
a = (4r/√3)
2. Calculating the Volume
The volume (V) of a cube is simply the cube of its edge length:
V = a³
Substituting the expression for 'a' in terms of 'r', we get:
V = ((4r/√3)³) = (64r³/3√3)
This is the general formula for calculating the volume of a BCC unit cell in terms of the atomic radius.
3. Practical Application and Example
Let's illustrate this with an example. Consider iron (Fe), which possesses a BCC structure. The atomic radius of iron is approximately 126 pm (picometers). Let's calculate the volume of the BCC unit cell for iron:
- Atomic radius (r) = 126 pm
- Volume (V) = (64r³/3√3)
- V = (64 * (126 pm)³ / (3√3))
- V ≈ 2.36 x 10⁶ pm³
This calculation provides the volume of a single BCC unit cell for iron. Remember to always use consistent units throughout your calculations. In this case, we use picometers (pm).
Beyond the Basics: Exploring Advanced Concepts
While the basic calculation outlined above provides a foundational understanding, there are several advanced concepts worth exploring:
1. Packing Efficiency
Packing efficiency refers to the percentage of the unit cell volume occupied by atoms. For a BCC unit cell, the packing efficiency is approximately 68%. This means that 68% of the unit cell's volume is occupied by atoms, while the remaining 32% is empty space. This lower packing efficiency compared to other structures like Face-Centered Cubic (FCC) highlights the differences in atomic arrangements and their impact on material properties.
2. Relationship to Density
The volume of the BCC unit cell is directly related to the density of the material. Knowing the volume of the unit cell and the mass of the atoms within it, one can calculate the density using the following formula:
Density (ρ) = (Mass of atoms in the unit cell) / (Volume of the unit cell)
This relationship is immensely valuable in materials science, as it allows for the determination of density from crystallographic data.
3. Applications in Materials Science and Engineering
Understanding the BCC unit cell volume is essential for numerous applications in materials science and engineering. The volume influences several key material properties, including:
- Mechanical strength: The specific arrangement of atoms in the BCC structure directly impacts its yield strength, tensile strength, and ductility.
- Thermal properties: The packing efficiency and interatomic spacing affect thermal conductivity and expansion.
- Electrical conductivity: The arrangement of atoms influences the ease with which electrons can move through the material.
- Magnetic properties: Some BCC metals exhibit magnetic properties, which are closely linked to their atomic structure.
The BCC structure is common in many transition metals like iron, chromium, molybdenum, and tungsten. Understanding its unit cell volume is critical for predicting and controlling the properties of materials containing these elements.
Conclusion: Mastering the BCC Unit Cell Volume Calculation
Mastering the calculation of the BCC unit cell volume provides a fundamental understanding of crystallography and its applications in materials science and engineering. This knowledge enables the prediction of material properties based on atomic structure, opening doors to material design and optimization. By understanding the relationship between atomic radius, edge length, and volume, along with the concepts of packing efficiency and density, you equip yourself with the tools necessary to analyze and manipulate the properties of a wide range of materials. The seemingly simple calculation of the BCC unit cell volume unlocks a world of complex material behavior and design possibilities. Remember to always practice your calculations and explore the rich tapestry of crystallography to deepen your understanding of this essential field.
Latest Posts
Latest Posts
-
15 Gallons Is How Many Liters
Mar 21, 2025
-
Which Two Planets Have No Moons
Mar 21, 2025
-
Common Factors Of 3 And 6
Mar 21, 2025
-
What Is The Highest Common Factor Of 12 And 4
Mar 21, 2025
-
Which Trait Is Polygenic In Humans
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Volume Of A Bcc Unit Cell . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
