Questions On Balancing Chemical Equations With Answers
Juapaving
Mar 20, 2025 · 5 min read
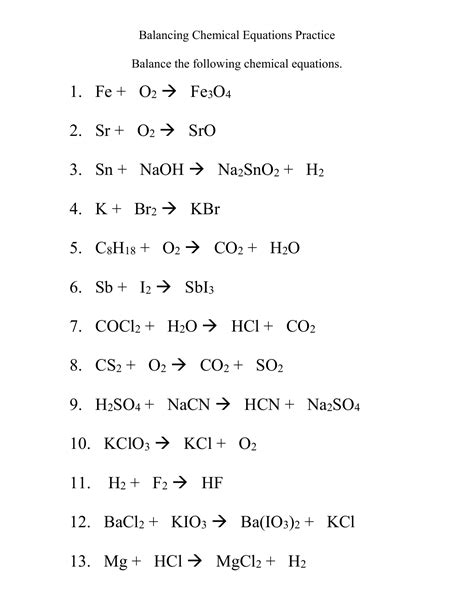
Table of Contents
Balancing Chemical Equations: A Comprehensive Guide with Questions and Answers
Balancing chemical equations is a fundamental skill in chemistry. It's crucial for understanding stoichiometry, predicting reaction yields, and accurately representing chemical processes. This comprehensive guide will delve into the techniques involved, address common challenges, and provide numerous practice questions with detailed solutions. Mastering this skill will significantly enhance your understanding of chemistry.
Why Balancing Chemical Equations is Important
Before diving into the mechanics, let's understand the significance of balanced chemical equations. The Law of Conservation of Mass dictates that matter cannot be created or destroyed in a chemical reaction. Therefore, the total mass of reactants must equal the total mass of products. A balanced equation reflects this fundamental law by ensuring that the number of atoms of each element is the same on both sides of the equation. An unbalanced equation is incomplete and doesn't accurately represent the reaction.
Consequences of Unbalanced Equations:
- Incorrect stoichiometric calculations: Unbalanced equations lead to inaccurate predictions of reactant and product amounts.
- Misinterpretation of reaction pathways: They fail to provide a true representation of the chemical changes occurring.
- Errors in experimental design: They can result in flawed experimental designs and unreliable results.
Methods for Balancing Chemical Equations
Several methods can be employed to balance chemical equations. The most common are:
1. Inspection Method (Trial and Error)
This method involves systematically adjusting coefficients until the number of atoms of each element is equal on both sides. It's best suited for simpler equations.
Example: Balance the equation: Fe + O₂ → Fe₂O₃
- Start with the most complex molecule: Fe₂O₃ contains two iron (Fe) atoms and three oxygen (O) atoms.
- Balance iron: Add a coefficient of 2 in front of Fe: 2Fe + O₂ → Fe₂O₃
- Balance oxygen: We now have three oxygen atoms on the right and two on the left. To balance, we need a coefficient of 3/2 in front of O₂: 2Fe + (3/2)O₂ → Fe₂O₃. However, coefficients should be whole numbers. Multiply the entire equation by 2 to eliminate the fraction: 4Fe + 3O₂ → 2Fe₂O₃
The balanced equation is: 4Fe + 3O₂ → 2Fe₂O₃
2. Algebraic Method
This method uses algebraic variables to represent the coefficients and solve for their values. It's particularly useful for more complex equations.
Example: Balance the equation: C₂H₆ + O₂ → CO₂ + H₂O
- Assign variables: Let's assign variables to the coefficients: aC₂H₆ + bO₂ → cCO₂ + dH₂O
- Write equations for each element:
- Carbon (C): 2a = c
- Hydrogen (H): 6a = 2d
- Oxygen (O): 2b = 2c + d
- Solve the system of equations: You can choose a value for one variable and solve for the others. Let's choose a = 1. Then:
- c = 2a = 2
- d = 3a = 3
- 2b = 2(2) + 3 = 7 => b = 7/2
- Eliminate fractions: Multiply the entire equation by 2: 2C₂H₆ + 7O₂ → 4CO₂ + 6H₂O
The balanced equation is: 2C₂H₆ + 7O₂ → 4CO₂ + 6H₂O
Practice Questions with Answers
Now let's work through some practice problems to solidify your understanding.
Question 1: Balance the following equation: Al + HCl → AlCl₃ + H₂
Answer: 2Al + 6HCl → 2AlCl₃ + 3H₂
Question 2: Balance the following equation: C₃H₈ + O₂ → CO₂ + H₂O
Answer: C₃H₈ + 5O₂ → 3CO₂ + 4H₂O
Question 3: Balance the following equation: Fe₂O₃ + CO → Fe + CO₂
Answer: Fe₂O₃ + 3CO → 2Fe + 3CO₂
Question 4: Balance the following equation: KClO₃ → KCl + O₂
Answer: 2KClO₃ → 2KCl + 3O₂
Question 5 (More Challenging): Balance the following equation: C₄H₁₀ + O₂ → CO₂ + H₂O
Answer: 2C₄H₁₀ + 13O₂ → 8CO₂ + 10H₂O
Question 6 (More Challenging): Balance the following equation: NH₃ + O₂ → NO + H₂O
Answer: 4NH₃ + 5O₂ → 4NO + 6H₂O
Question 7 (Involving Polyatomic Ions): Balance the equation: Ca(OH)₂ + H₃PO₄ → Ca₃(PO₄)₂ + H₂O
Answer: 3Ca(OH)₂ + 2H₃PO₄ → Ca₃(PO₄)₂ + 6H₂O
Question 8 (Redox Reaction): Balance the following redox reaction in acidic solution: MnO₄⁻ + Fe²⁺ → Mn²⁺ + Fe³⁺
Answer: (This requires a more advanced balancing technique, often using the half-reaction method. The balanced equation in acidic solution is: 8H⁺ + MnO₄⁻ + 5Fe²⁺ → Mn²⁺ + 5Fe³⁺ + 4H₂O)
Question 9 (Combustion Reaction): Balance the combustion reaction of propane (C₃H₈) in air.
Answer: C₃H₈ + 5O₂ → 3CO₂ + 4H₂O
Question 10 (Neutralization Reaction): Balance the neutralization reaction between sulfuric acid (H₂SO₄) and potassium hydroxide (KOH).
Answer: H₂SO₄ + 2KOH → K₂SO₄ + 2H₂O
Tips and Tricks for Balancing Equations
- Start with the most complex molecule: Balancing the most complex molecule first often simplifies the process.
- Balance polyatomic ions as units: If a polyatomic ion appears unchanged on both sides, treat it as a single unit.
- Check your work: After balancing, always double-check that the number of atoms of each element is the same on both sides.
- Practice regularly: The more you practice, the easier it will become.
- Use different methods: Try both the inspection and algebraic methods to find the approach that suits you best.
- Don't be afraid to start over: If you get stuck, don't hesitate to start again with a fresh approach.
Beyond Balancing: Stoichiometry and Beyond
Mastering the art of balancing chemical equations is just the first step in understanding stoichiometry, a crucial area of chemistry. Stoichiometry allows you to calculate the amounts of reactants and products involved in a chemical reaction, based on the balanced equation. This knowledge extends to applications in various fields, including:
- Industrial chemistry: Optimizing reaction yields and minimizing waste.
- Environmental science: Analyzing pollutants and predicting their environmental impact.
- Pharmaceutical chemistry: Determining the correct dosages of medications.
- Material science: Designing new materials with specific properties.
By developing a strong foundation in balancing chemical equations, you're setting yourself up for success in more advanced chemical concepts and applications. Remember to practice consistently and don't hesitate to seek help if needed. The key is persistence and a systematic approach.
Latest Posts
Latest Posts
-
Equation Of A Plane Given 3 Points
Mar 21, 2025
-
Why Does Temperature Stay Constant During A Phase Change
Mar 21, 2025
-
What Is The Freezing Point Of Fahrenheit
Mar 21, 2025
-
Is Ba Oh 2 Ionic Or Molecular
Mar 21, 2025
-
Does A Liquid Have A Definite Volume
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Questions On Balancing Chemical Equations With Answers . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
