The Si Unit Of Work Is
Juapaving
Mar 23, 2025 · 6 min read
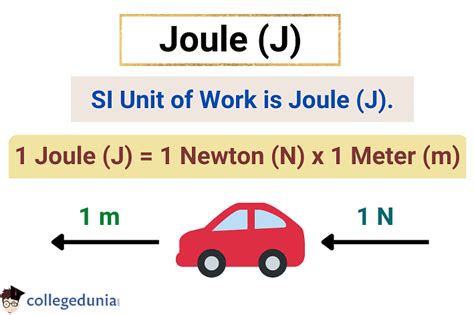
Table of Contents
The SI Unit of Work Is: A Deep Dive into Joules and Their Applications
The SI unit of work is the joule (J). This seemingly simple statement belies a rich and multifaceted concept fundamental to physics, engineering, and numerous other scientific disciplines. Understanding the joule, its definition, and its applications is crucial for grasping the principles governing energy transfer and transformation. This article delves deep into the joule, exploring its definition, derivations, practical applications, and the broader implications of work in various fields.
Defining Work in Physics
Before we delve into the specifics of the joule, let's establish a clear understanding of what constitutes "work" in physics. Unlike its everyday meaning, which often implies mental or physical exertion, work in physics has a precise definition:
Work is done when a force causes an object to move in the direction of the force. This is a crucial point: the force must be applied in the same direction as the displacement of the object. If the force is perpendicular to the displacement (like carrying a weight horizontally), no work is done in the physics sense, regardless of the effort exerted.
This definition leads us to the mathematical formula for work:
Work (W) = Force (F) x Distance (d) x cos(θ)
where θ is the angle between the force vector and the displacement vector. When the force and displacement are in the same direction (θ = 0°), the equation simplifies to:
W = Fd
The Joule: A Measure of Energy Transfer
The joule, named after the 19th-century physicist James Prescott Joule, quantifies the amount of work done. One joule is defined as the work done when a force of one newton (N) acts over a distance of one meter (m) in the same direction. Therefore:
1 Joule (J) = 1 Newton-meter (N⋅m)
This seemingly simple definition has profound implications, connecting mechanics, thermodynamics, and other branches of physics. The joule isn't just a measure of mechanical work; it's a fundamental unit of energy. Any form of energy, whether kinetic, potential, thermal, electrical, or chemical, can be expressed in joules. This unification is a cornerstone of the SI system, emphasizing the interconnectedness of various energy forms.
Deriving the Joule from Fundamental SI Units
The joule's definition is built upon the fundamental SI units of mass (kilogram, kg), length (meter, m), and time (second, s). Let's break down this derivation:
-
Newton (N): The newton, the SI unit of force, is derived from Newton's second law of motion (F = ma), where 'm' represents mass (kg) and 'a' represents acceleration (m/s²). Thus, 1 N = 1 kg⋅m/s².
-
Joule (J): Substituting the definition of the newton into the work equation (W = Fd), we get: 1 J = 1 (kg⋅m/s²)⋅m = 1 kg⋅m²/s².
This shows that the joule is fundamentally derived from the base SI units of mass, length, and time. This consistency and interrelation between units are hallmarks of the SI system's elegance and practicality.
Applications of the Joule Across Disciplines
The joule's versatility is evident in its widespread application across various scientific and engineering domains:
1. Mechanics:
-
Calculating work done by machines: From simple levers and pulleys to complex engines, the joule allows engineers to quantify the mechanical work performed. This is critical for efficiency analyses, power calculations, and designing more efficient systems.
-
Determining kinetic and potential energy: The joule measures both kinetic energy (energy of motion) and potential energy (stored energy). Understanding these energy forms is essential in designing roller coasters, predicting projectile motion, and analyzing energy transformations in various systems.
-
Analyzing collisions: The principle of conservation of energy, expressed in joules, governs collisions, enabling physicists to analyze the energy transfer during impact.
2. Thermodynamics:
-
Measuring heat energy: Heat is a form of energy, and the joule is the unit used to quantify it. Thermodynamic processes, such as heating and cooling, are quantified in joules, facilitating calculations related to energy transfer and efficiency in engines, power plants, and industrial processes.
-
Determining internal energy changes: The joule helps in analyzing internal energy changes within systems, providing insights into the energy content and transformations within those systems.
3. Electricity and Magnetism:
-
Quantifying electrical energy: Electrical energy, whether in batteries, power grids, or electronic circuits, is measured in joules. This enables calculations related to power consumption, energy storage, and efficiency in electrical systems.
-
Determining the work done by electromagnetic forces: Electromagnetic forces, such as those involved in electric motors and generators, also perform work measured in joules.
4. Nuclear Physics:
- Measuring energy released in nuclear reactions: Nuclear reactions, like fission and fusion, release tremendous amounts of energy, typically expressed in joules. Understanding this energy release is crucial for nuclear power generation and the study of nuclear weapons.
5. Chemistry:
-
Determining energy changes in chemical reactions: Chemical reactions involve energy changes, either released (exothermic) or absorbed (endothermic). These energy changes are expressed in joules, allowing chemists to understand reaction energetics and predict reaction spontaneity.
-
Measuring bond energies: The strength of chemical bonds is measured in joules, enabling prediction of reaction rates and stability.
Beyond the Joule: Related Units and Conversions
While the joule is the primary unit of work and energy, several related units are commonly used, particularly for expressing larger or smaller quantities:
- Kilojoule (kJ): 1 kJ = 1000 J
- Megajoule (MJ): 1 MJ = 1,000,000 J
- Gigajoule (GJ): 1 GJ = 1,000,000,000 J
- Terajoule (TJ): 1 TJ = 1,000,000,000,000 J
These prefixes simplify the expression of very large energy values often encountered in fields like power generation and large-scale industrial processes. Conversely, smaller units like millijoules (mJ) and microjoules (µJ) are used to describe energy in smaller-scale applications.
Converting between these units involves simple multiplication or division by powers of 10, based on the standard metric prefixes. For instance, converting 5000 J to kJ involves dividing by 1000, resulting in 5 kJ.
The Joule and Power
Closely related to work is the concept of power, which represents the rate at which work is done or energy is transferred. The SI unit of power is the watt (W), defined as one joule per second (J/s). Therefore:
1 Watt (W) = 1 Joule/second (J/s)
Power calculations are crucial in determining the efficiency of engines, evaluating the performance of electrical appliances, and understanding energy consumption rates in various systems. The watt and its multiples (kilowatts, megawatts, etc.) are commonly used in electrical engineering and power systems.
Conclusion
The joule, as the SI unit of work, is far more than a simple unit of measurement. It's a fundamental cornerstone of physics, representing a unifying concept that links mechanics, thermodynamics, electricity, and other fields. Its application spans a vast range of disciplines, from designing efficient machines to understanding nuclear reactions. By mastering the concept of the joule and its applications, we gain a deeper appreciation for the fundamental principles governing energy and its transformations in the universe around us. Understanding the joule's derivation from fundamental SI units further emphasizes the elegance and interconnectedness inherent in the system of units we use to quantify the physical world. The widespread use of the joule and its related units demonstrates its importance in both theoretical and practical applications of physics and engineering. Its simple definition belies its powerful applications across diverse scientific endeavors.
Latest Posts
Latest Posts
-
Is Cooking A Chemical Or Physical Change
Mar 25, 2025
-
Is Concave Mirror Converging Or Diverging
Mar 25, 2025
-
How To Find Orthocentre Of A Triangle
Mar 25, 2025
-
7 Continents And 5 Oceans List
Mar 25, 2025
-
Dissolving Of Salt In Water Physical Or Chemical
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about The Si Unit Of Work Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
