Is Cooking A Chemical Or Physical Change
Juapaving
Mar 25, 2025 · 6 min read
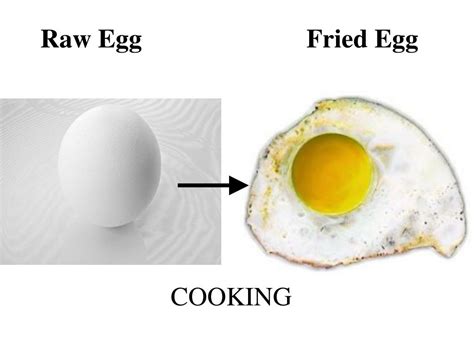
Table of Contents
Is Cooking a Chemical or Physical Change? A Deep Dive into Kitchen Chemistry
Cooking, a fundamental human activity, is far more than just combining ingredients and applying heat. It's a fascinating interplay of chemical and physical transformations, a culinary dance between molecules and matter. This article delves deep into the science behind cooking, exploring the chemical and physical changes involved in various cooking methods and revealing the intricacies of this everyday process. We'll uncover why understanding these changes is crucial for achieving culinary perfection.
The Fundamentals: Chemical vs. Physical Changes
Before diving into the complexities of cooking, it's essential to understand the core difference between chemical and physical changes.
Physical changes alter the form or appearance of a substance without changing its chemical composition. Think of cutting an apple – you've changed its shape, but it's still an apple. Other examples include melting ice (water changes state but remains H₂O), dissolving sugar in water (sugar particles disperse, but the sugar remains sugar), and boiling water (water changes state, but remains water). These changes are often reversible.
Chemical changes, also known as chemical reactions, involve a rearrangement of atoms and molecules, resulting in the formation of new substances with different properties. These changes are often irreversible. Examples include burning wood (wood transforms into ash and gases), rusting iron (iron reacts with oxygen to form iron oxide), and baking a cake (ingredients undergo chemical reactions to form a new structure).
Cooking: A Symphony of Chemical and Physical Transformations
Cooking is a complex process involving both chemical and physical changes, often occurring simultaneously. The relative contribution of each depends heavily on the cooking method and the ingredients involved.
Physical Changes in Cooking
Many initial steps in cooking involve physical changes:
- Chopping vegetables: This purely alters the physical form of the vegetables; their chemical composition remains unchanged.
- Mixing ingredients: This combines different substances without altering their chemical structure. For instance, mixing flour, sugar, and eggs doesn't create new chemical compounds.
- Dissolving salt in water: The salt molecules disperse in the water, but the salt remains chemically unchanged.
- Boiling water: This changes water from liquid to gas (steam), a physical change of state, but it remains water (H₂O).
- Melting butter or chocolate: These substances change from solid to liquid, but their chemical composition stays the same.
- Freezing: This is a physical change of state, where the molecules slow down and form a solid structure.
Chemical Changes in Cooking
The real magic of cooking lies in the chemical reactions that transform ingredients. These reactions are often catalyzed by heat:
-
Maillard reaction: This is arguably the most significant chemical reaction in cooking. It occurs when amino acids and reducing sugars react at high temperatures (around 140-165°C or 284-329°F), creating hundreds of different flavor and aroma compounds. This reaction is responsible for the browning and delicious flavors of roasted meats, seared steaks, and baked goods. It's fundamentally irreversible.
-
Caramelization: This is the browning of sugars when heated to high temperatures (above 160-180°C or 320-356°F). This process creates a complex array of compounds that contribute to sweet, caramel-like flavors and aromas. Like the Maillard reaction, it's irreversible.
-
Protein denaturation: Proteins, the building blocks of many foods (meat, eggs, etc.), have a complex 3D structure. Heat disrupts these structures, causing them to unfold (denature). This change is primarily physical initially but often leads to further chemical changes, altering texture and digestibility. For example, cooking an egg causes the proteins to denature, resulting in a solidified egg white and yolk. While denaturation is often reversible under certain conditions, in the context of cooking, it is generally irreversible.
-
Starch gelatinization: Starch granules, found in many foods (potatoes, rice, pasta), swell and absorb water when heated. This creates a thicker, more viscous texture. This is a physical change initially but involves some chemical changes in the starch structure, making it irreversible.
-
Enzyme activity: Many enzymes in foods are deactivated by heat. This can improve texture and prevent undesirable changes, such as enzymatic browning in fruits. Enzyme deactivation is an irreversible chemical change.
-
Lipid oxidation: Fats and oils can undergo oxidation, a chemical reaction with oxygen, resulting in rancidity. High temperatures and prolonged cooking times can accelerate this process, negatively impacting the flavor and aroma of the food. This is an irreversible chemical change.
-
Acid-base reactions: Adding acidic ingredients like lemon juice or vinegar can alter the pH of a dish, influencing various chemical reactions and the overall flavor profile. This is a chemical change.
Cooking Methods and Their Chemical and Physical Transformations
Let's examine how different cooking methods contribute to these changes:
Baking: Baking primarily involves heat transfer through conduction and convection. This leads to significant chemical changes like the Maillard reaction and caramelization, responsible for the browning and flavor development in baked goods. Physical changes include starch gelatinization in cakes and breads.
Boiling: Boiling involves heating a liquid until it reaches its boiling point. While the water undergoes a physical change of state (liquid to gas), the food immersed in the boiling water undergoes both physical (e.g., softening of vegetables) and chemical changes (e.g., protein denaturation).
Frying: Frying uses hot oil to transfer heat through conduction and convection. The high temperatures involved promote the Maillard reaction and caramelization, contributing significantly to the flavor and browning of the food. Physical changes include the cooking of the outer surface while the inside may still be undercooked.
Roasting: Roasting is a dry-heat cooking method that typically involves high temperatures and a longer cooking time. The Maillard reaction and caramelization are prominent, resulting in deeply browned and flavorful food. Significant physical and chemical changes occur within the food, especially protein denaturation and the alteration of moisture content.
Grilling: Grilling uses direct, high heat often resulting in charring and a smoky flavor profile. The Maillard reaction is crucial here, contributing to the rich flavors and unique texture.
Steaming: Steaming uses moist heat to gently cook food. While there are some chemical changes, like protein denaturation, steaming is mainly a process dominated by physical changes, with limited browning or flavor changes from reactions like the Maillard reaction.
The Importance of Understanding Chemical and Physical Changes in Cooking
Understanding the chemical and physical changes during cooking is crucial for:
- Achieving desired texture: Knowing how heat affects proteins and starches allows for precise control of the final texture of the food.
- Developing optimal flavors: Understanding the Maillard reaction and caramelization helps to maximize the flavor development of dishes.
- Preventing undesirable changes: Knowledge of lipid oxidation and enzyme activity helps in preventing spoilage and undesirable flavor changes.
- Improving cooking efficiency: Understanding the processes involved can optimize cooking times and techniques.
- Creating innovative culinary experiences: A strong understanding of the chemistry and physics of cooking unlocks the potential for creativity and innovation in the kitchen.
Conclusion: Cooking – A Science and an Art
Cooking is a fascinating blend of science and art. While the art lies in creativity, technique, and personal touch, the science underpins the fundamental transformations that occur when we cook. By understanding the chemical and physical changes involved, we can elevate our culinary skills, create more flavorful and delicious dishes, and appreciate the intricate interplay of molecules that transforms raw ingredients into culinary masterpieces. The journey of understanding cooking's science is a continuous one, rich in discovery and rewarding in its results. Embrace this journey and your culinary creations will undoubtedly benefit.
Latest Posts
Latest Posts
-
1 Square Mile Is How Many Acres
Mar 26, 2025
-
What Is The Boiling Point And Freezing Point Of Water
Mar 26, 2025
-
What Is A Multiple Of 25
Mar 26, 2025
-
Are Multiples Of 4 Always Even
Mar 26, 2025
-
Triangle With Two Equal Sides Is Called
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Is Cooking A Chemical Or Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
