The Most Abundant Metal In Earth's Crust Is
Juapaving
Mar 30, 2025 · 6 min read
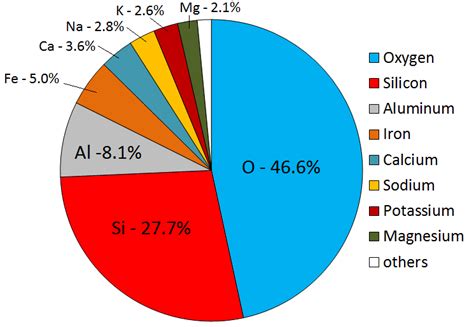
Table of Contents
The Most Abundant Metal in Earth's Crust: Unveiling the Secrets of Aluminum
Aluminum. The name might conjure images of sleek smartphones, lightweight soda cans, or even the foil clinging to your leftover dinner. But beyond its ubiquitous presence in modern life, aluminum holds a far more significant role: it's the most abundant metal in Earth's crust. This seemingly simple fact unlocks a wealth of fascinating geological, industrial, and even historical information. This article delves deep into the world of aluminum, exploring its abundance, its properties, its extraction, its applications, and its future prospects.
The Astonishing Abundance of Aluminum
While often associated with modern technology, aluminum's story began billions of years ago, locked within the Earth's crust. It makes up a staggering 8.23% of the Earth's crust by weight, dwarfing the abundance of other metals like iron (5%), titanium (0.6%), and manganese (0.1%). This extraordinary prevalence underscores its importance in understanding Earth's geological processes and the formation of our planet.
Why So Abundant?
Aluminum's dominance isn't accidental. Its genesis lies in the processes that shaped our planet. During the Earth's formation, various elements sorted themselves based on their density and chemical properties. Aluminum, with its relatively light atomic weight, became concentrated in the Earth's crust. Further geological processes, such as weathering and erosion, continued to redistribute aluminum-containing minerals, further increasing its prevalence in accessible deposits.
Key Aluminum-Bearing Minerals
Aluminum rarely exists in its pure metallic form in nature. Instead, it's found predominantly within various minerals, the most important of which is bauxite. Bauxite is a sedimentary rock rich in aluminum hydroxides, specifically gibbsite, boehmite, and diaspore. Other important aluminum-containing minerals include:
- Feldspar: A group of abundant rock-forming minerals found in many igneous and metamorphic rocks.
- Clay minerals: A diverse group of hydrous aluminum phyllosilicates formed from the weathering of other minerals.
- Micas: A group of sheet silicate minerals that often contain aluminum.
The widespread presence of these minerals throughout the Earth's crust directly contributes to aluminum's overall abundance.
Unveiling Aluminum's Properties: A Versatile Metal
Aluminum's dominance isn't solely due to its abundance; its remarkable properties also contribute to its widespread use. It boasts a unique combination of characteristics that make it exceptionally versatile:
-
Lightweight: Its low density makes it significantly lighter than other common metals like steel and iron, making it ideal for applications requiring portability and fuel efficiency.
-
High Strength-to-Weight Ratio: Despite its lightness, aluminum possesses surprisingly high strength, particularly when alloyed with other elements. This allows for the construction of strong yet lightweight structures.
-
Excellent Corrosion Resistance: Aluminum's natural tendency to form a protective oxide layer on its surface safeguards it from corrosion, enhancing its durability and longevity.
-
High Conductivity: Aluminum is a good conductor of both electricity and heat, making it suitable for electrical wiring, cookware, and heat exchangers.
-
Malleability and Ductility: Aluminum is easily shaped and molded, making it highly amenable to various manufacturing processes.
-
Recyclability: Aluminum is infinitely recyclable without losing its properties, making it an environmentally friendly material.
From Bauxite to Ingot: Extracting Aluminum – A Technological Marvel
While aluminum is abundant, extracting it from its ore requires a significant technological feat. The process, known as the Bayer process, is a crucial step in obtaining pure aluminum. It involves several key stages:
1. Bauxite Mining and Purification:
The journey begins with mining bauxite ore from open-pit mines. The raw ore is then purified to remove impurities like silica and iron oxides. This purification is crucial for efficient aluminum extraction.
2. Digestion:
The purified bauxite is digested under pressure with a hot caustic soda solution (sodium hydroxide). This process dissolves the aluminum hydroxide, leaving behind the insoluble impurities.
3. Precipitation:
After separating the impurities, the aluminum hydroxide is precipitated from the solution by carefully controlling the pH and temperature. This results in a white, gelatinous precipitate of aluminum hydroxide.
4. Calcination:
The aluminum hydroxide is then calcined (heated to high temperatures) to produce alumina (aluminum oxide), a white powder. This alumina is the primary feedstock for aluminum smelting.
5. The Hall-Héroult Process:
This electrolytic process is the final step in producing pure aluminum. Alumina is dissolved in molten cryolite, an aluminum fluoride mineral, and then subjected to electrolysis. This process involves passing a strong electric current through the molten mixture, causing the aluminum ions to be reduced to metallic aluminum at the cathode (negative electrode).
A World of Applications: Aluminum's Impact on Modern Life
The versatility of aluminum is reflected in its extensive range of applications across diverse industries:
Transportation:
-
Automotive: Aluminum alloys are increasingly used in vehicle bodies, chassis components, and engine parts to reduce weight and improve fuel efficiency.
-
Aerospace: Aluminum's lightweight strength makes it a vital material in aircraft construction, reducing fuel consumption and enhancing performance.
-
Rail: Aluminum is used in high-speed trains and railway components for their lightweight and corrosion resistance.
Packaging:
-
Beverage cans: Aluminum cans are lightweight, recyclable, and offer excellent protection against oxidation.
-
Food containers: Aluminum foil and containers are used extensively for food storage and preservation.
Construction:
-
Building materials: Aluminum is used in window frames, doors, cladding, and roofing materials due to its corrosion resistance and ease of fabrication.
-
Structural components: Aluminum alloys are employed in bridges and other structures requiring high strength and low weight.
Electrical Engineering:
-
Transmission lines: Aluminum's high electrical conductivity makes it ideal for high-voltage transmission lines.
-
Electrical components: Aluminum is used in various electronic components, including capacitors and resistors.
Consumer Goods:
-
Household appliances: Aluminum is frequently used in kitchenware, appliances, and other household items.
-
Sporting goods: Aluminum's lightweight strength is advantageous in sporting equipment like bicycles, bats, and racquets.
Environmental Considerations and the Future of Aluminum
Despite its remarkable properties, the aluminum industry faces environmental challenges:
-
Bauxite mining: Open-pit mining can lead to habitat destruction and soil erosion.
-
Energy consumption: The Hall-Héroult process is energy-intensive, contributing to greenhouse gas emissions.
-
Waste management: Proper management of bauxite residue (red mud), a byproduct of the Bayer process, is crucial to mitigate environmental risks.
However, ongoing research and technological advancements aim to mitigate these environmental concerns:
-
Improved energy efficiency: Innovations in the smelting process are reducing energy consumption.
-
Recycling initiatives: Recycling aluminum significantly reduces the need for bauxite mining and energy consumption.
-
Sustainable mining practices: Implementing responsible mining practices minimizes environmental impact.
The future of aluminum hinges on finding sustainable solutions that balance its economic value with its environmental impact.
Conclusion: A Metal for the Ages
Aluminum's position as the most abundant metal in Earth's crust is not merely a geological curiosity; it's a cornerstone of our modern world. Its unique properties, combined with its relative abundance and recyclability, have propelled it into a vast array of applications, shaping industries and improving our lives. However, addressing the environmental challenges associated with aluminum production and consumption remains crucial for ensuring its sustainable use in the years to come. As we continue to innovate and develop more efficient and environmentally friendly processes, aluminum's vital role in shaping our future will undoubtedly persist.
Latest Posts
Latest Posts
-
Differences Between Primary Data And Secondary Data
Apr 01, 2025
-
Where Glucose Gets Broken Into Pyruvate In The Cell
Apr 01, 2025
-
Find The Inverse Of The Relation
Apr 01, 2025
-
How Are Cellular Respiration And Photosynthesis Related
Apr 01, 2025
-
How Many Rna Polymerases Are Found In Prokaryotes
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about The Most Abundant Metal In Earth's Crust Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
