The Mass Of A Substance Per Unit Volume
Juapaving
Mar 14, 2025 · 5 min read
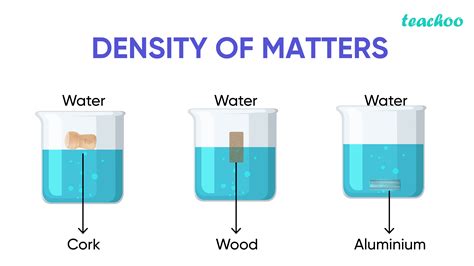
Table of Contents
The Mass of a Substance Per Unit Volume: Density and its Applications
Density, the mass of a substance per unit volume, is a fundamental physical property with far-reaching implications across various scientific disciplines and everyday life. Understanding density allows us to predict the behavior of materials, design efficient systems, and even unravel mysteries of the universe. This comprehensive exploration delves into the intricacies of density, examining its definition, calculation, units, variations, applications, and significance.
Defining Density: Mass Divided by Volume
Density (ρ, pronounced "rho") is defined as the ratio of an object's mass (m) to its volume (V). In simpler terms, it tells us how much matter is packed into a given space. The formula is elegantly simple:
ρ = m/V
This seemingly straightforward equation holds profound implications. A high density indicates a large amount of mass concentrated in a small volume, while a low density suggests the opposite. For instance, lead has a high density, meaning a small piece of lead is surprisingly heavy, while air has a low density, meaning a large volume of air weighs relatively little.
Units of Density
The units of density depend on the units used for mass and volume. The most common unit in the International System of Units (SI) is kilograms per cubic meter (kg/m³). Other commonly used units include:
- grams per cubic centimeter (g/cm³): Often preferred in chemistry and materials science due to its convenient scale. Note that 1 g/cm³ is exactly equal to 1000 kg/m³.
- pounds per cubic foot (lb/ft³): Commonly used in engineering applications in the United States.
- grams per milliliter (g/mL): Equivalent to g/cm³, often used for liquids.
Calculating Density: A Practical Approach
Calculating the density of a substance requires determining both its mass and volume. Mass is typically measured using a balance or scale. Volume determination, however, can be more complex and depends on the state of the substance:
Measuring Volume of Solids:
- Regularly shaped solids: For objects with well-defined geometric shapes (cubes, spheres, cylinders), the volume can be calculated using standard geometric formulas.
- Irregularly shaped solids: The volume of irregularly shaped solids is often determined using water displacement. The object is submerged in a known volume of water, and the increase in water level represents the object's volume.
Measuring Volume of Liquids:
Liquids are typically measured using graduated cylinders, beakers, or volumetric flasks, depending on the required precision.
Measuring Volume of Gases:
Gas volume is highly sensitive to changes in temperature and pressure. Therefore, the measurement of gas volume requires careful consideration of these factors, often using specialized equipment like gas burettes or gasometers. The ideal gas law (PV = nRT) provides a fundamental relationship for determining gas volume.
Density Variations: Factors Influencing Density
The density of a substance is not always constant. Several factors can influence its value:
- Temperature: Generally, as temperature increases, the volume of a substance expands, leading to a decrease in density. This is because the mass remains constant while the volume increases. Water is a notable exception to this rule, exhibiting a unique density anomaly near its freezing point.
- Pressure: Increasing pressure compresses a substance, reducing its volume and thus increasing its density. This effect is more pronounced in gases than in solids or liquids.
- Composition: The density of a mixture or solution depends on the densities and proportions of its constituent components.
- Phase: The density of a substance varies depending on its phase (solid, liquid, or gas). Generally, solids are denser than liquids, and liquids are denser than gases.
Applications of Density: A Wide Spectrum of Uses
The concept of density finds applications across a wide range of fields:
In Science and Engineering:
- Material identification: Density is a crucial characteristic used to identify unknown materials. Comparing the measured density of a sample to known values in a database can help determine its composition.
- Fluid mechanics: Density is a fundamental parameter in fluid mechanics, influencing buoyancy, pressure distribution, and flow patterns. Archimedes' principle, which describes buoyancy, directly relies on density differences.
- Geophysics: Density variations within the Earth's interior provide valuable insights into its structure and composition. Geophysicists use density measurements to understand tectonic plate movements and locate valuable mineral resources.
- Astronomy: The density of stars and planets is crucial in understanding their formation, evolution, and internal structure. Density measurements help determine the composition and age of celestial bodies.
- Aerospace Engineering: Aircraft design and flight dynamics rely heavily on understanding density variations in the atmosphere. Changes in air density with altitude directly impact lift and drag forces.
In Everyday Life:
- Separation of mixtures: Density differences are frequently exploited to separate mixtures. For instance, panning for gold utilizes the density difference between gold and other materials. Centrifugation, a laboratory technique, also relies on density differences to separate components in a mixture.
- Buoyancy: The principle of buoyancy, directly related to density, explains why objects float or sink. Ships float because their average density is less than the density of water.
- Hydrometry: The measurement of the density of liquids, particularly water, is crucial in various applications, including determining water purity and salinity. Hydrometers are specifically designed for this purpose.
Relative Density and Specific Gravity: Related Concepts
Relative density and specific gravity are closely related to density, providing alternative ways to express the density of a substance:
- Relative density: The ratio of the density of a substance to the density of a reference substance. Water is commonly used as the reference substance for solids and liquids.
- Specific gravity: Essentially the same as relative density, but often used more specifically for liquids.
Conclusion: Density – A Cornerstone of Understanding the Physical World
Density, the mass of a substance per unit volume, is a cornerstone of our understanding of the physical world. Its simple definition belies its profound implications across numerous scientific disciplines and everyday life. From identifying unknown materials to understanding the behavior of stars, density plays a critical role in our ability to interpret and interact with the universe around us. This comprehensive overview has touched upon the key aspects of density, including its definition, calculation, units, variations, and wide-ranging applications. Understanding density is not just a matter of academic interest; it is a fundamental concept with practical consequences and applications impacting our daily lives in countless ways. Further exploration into the nuances of density will continue to yield valuable insights and drive innovation across various fields.
Latest Posts
Latest Posts
-
Whats The Difference Between Alternator And Generator
Mar 14, 2025
-
What Does Xlv Mean In Roman Numbers
Mar 14, 2025
-
Is Melting Ice Chemical Or Physical Change
Mar 14, 2025
-
How Many Valence Electrons Are In Strontium
Mar 14, 2025
-
Is Gold A Mixture Or Pure Substance
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about The Mass Of A Substance Per Unit Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
