Is Gold A Mixture Or Pure Substance
Juapaving
Mar 14, 2025 · 6 min read
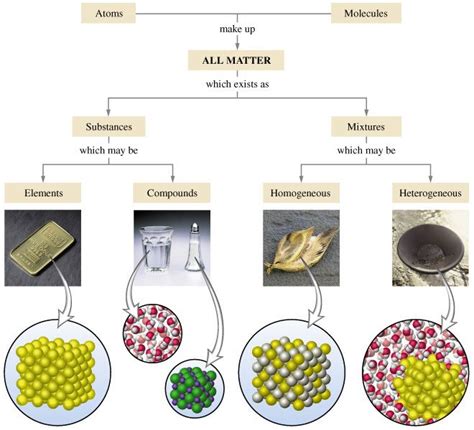
Table of Contents
Is Gold a Mixture or a Pure Substance? Unraveling the Nature of Gold
Gold, a precious metal coveted for millennia, holds a fascinating place in both human history and the scientific world. Its inherent beauty and valuable properties have driven its exploration and use for thousands of years. But from a scientific perspective, a fundamental question arises: is gold a mixture or a pure substance? This article delves deep into the chemical composition of gold, exploring its elemental nature and clarifying its classification within the scientific framework of matter. We will examine the properties that define gold as a pure substance and address any potential misconceptions surrounding its purity.
Understanding the Definitions: Mixture vs. Pure Substance
Before we delve into the specifics of gold, let's establish a clear understanding of the key terms: mixture and pure substance.
Pure Substance: A pure substance is a form of matter that has a constant chemical composition and possesses distinct chemical properties. It cannot be separated into simpler components through physical methods like filtration or distillation. Pure substances can be further categorized into elements and compounds.
-
Elements: Elements are the fundamental building blocks of matter, consisting of only one type of atom. They cannot be broken down into simpler substances by chemical means. Examples include gold (Au), oxygen (O), and hydrogen (H).
-
Compounds: Compounds are formed when two or more elements chemically combine in fixed proportions. They can only be separated into their constituent elements through chemical reactions. Examples include water (H₂O) and table salt (NaCl).
Mixture: A mixture is a combination of two or more substances that are not chemically bonded. The components retain their individual chemical properties and can be separated using physical methods. Mixtures can be homogeneous (uniform composition throughout) or heterogeneous (non-uniform composition).
Gold: An Elemental Pure Substance
Gold, represented by the chemical symbol Au (from the Latin word "aurum"), is unequivocally classified as a pure substance, specifically an element. This means it consists entirely of gold atoms, each containing 79 protons in its nucleus. These atoms are identical in terms of their atomic number and electronic configuration.
The characteristic properties we associate with gold—its lustrous yellow color, malleability, ductility, and high density—are direct consequences of its atomic structure and electronic configuration. These properties are consistent throughout a sample of pure gold, regardless of its origin or form.
Why Gold isn't a Mixture
The misconception that gold might be a mixture often arises from the presence of impurities in naturally occurring gold. Gold found in nature is rarely 100% pure; it usually exists as an alloy, mixed with other elements like silver, copper, and traces of other metals. However, this does not change the fundamental nature of gold itself. The presence of these impurities simply means that the gold sample is not pure gold, but rather a mixture containing gold.
The key distinction lies in the chemical bonds. In a mixture, the components are physically mixed but retain their individual identities and properties. The silver, copper, and other elements present in naturally occurring gold are not chemically bonded to the gold atoms; they are simply interspersed within the gold structure. These impurities can be removed through processes like refining, resulting in a higher purity of gold.
The Refining Process: Separating Gold from Impurities
The refining process relies on exploiting the different physical and chemical properties of gold and its associated impurities. Methods employed include:
-
Cyanide leaching: This process uses a cyanide solution to dissolve gold from its ore. The gold is then extracted from the solution through electrolysis.
-
Amalgamation: This traditional method uses mercury to dissolve gold. The mercury is later removed through heating, leaving behind the refined gold.
-
Electrorefining: This electrochemical process uses an electric current to purify gold, separating it from impurities.
These processes demonstrate that the impurities are not intrinsically part of the gold itself; they are separable through physical or chemical means.
Characteristics of Pure Gold (24 Karat Gold)
Pure gold, also known as 24-karat gold, exhibits unique properties:
- Malleability: It can be easily hammered or rolled into thin sheets.
- Ductility: It can be drawn into thin wires.
- High Density: It is a very dense metal.
- High Conductivity: It's an excellent conductor of both heat and electricity.
- Inertness: It is relatively unreactive, meaning it doesn't readily react with most chemicals.
- Lustrous Yellow Color: Its characteristic yellow color is unmistakable.
These properties are inherent to the gold element itself and are not dependent on the presence of other elements. The slight variations in these properties observed in different gold samples are due to the presence of impurities, not a change in the intrinsic nature of gold.
Gold Alloys: Mixtures Containing Gold
While pure gold is an element, it's rarely used in its pure form for practical applications. The softness of pure gold makes it unsuitable for many purposes. Therefore, gold is often alloyed with other metals to enhance its hardness, durability, and color. These alloys are mixtures, and their properties vary depending on the composition of the alloy.
Common gold alloys include:
- 18-karat gold: Typically contains 75% gold and 25% other metals, such as copper, silver, or zinc.
- 14-karat gold: Typically contains 58.3% gold and 41.7% other metals.
- 10-karat gold: Typically contains 41.7% gold and 58.3% other metals.
The karat system indicates the proportion of gold in an alloy. 24-karat gold represents pure gold, while lower karat numbers indicate a lower percentage of gold and a higher percentage of other metals. These alloys are mixtures because the gold and other metals are physically mixed but not chemically bonded.
Addressing Common Misconceptions
Several misconceptions surround the nature of gold:
-
Gold is always yellow: While pure gold is yellow, alloys of gold can exhibit a range of colors, from white to red, depending on the alloying metals.
-
All gold is valuable: The value of gold is primarily determined by its purity (karat) and market price. The presence of significant impurities reduces the value of the gold.
-
Gold never tarnishes: While gold is relatively inert, it can react with certain chemicals over extended periods. However, its resistance to tarnishing is significantly higher than many other metals.
Conclusion: Gold's Elemental Purity
In conclusion, gold (Au) is fundamentally a pure substance, an element consisting of only gold atoms. The presence of impurities in naturally occurring gold does not change its elemental nature; it simply means the sample is not pure gold but rather a mixture containing gold. The refining process effectively separates these impurities, yielding pure gold, highlighting the inherent difference between a pure substance and a mixture. The use of gold in alloys for practical applications creates mixtures that combine the valuable properties of gold with the enhancing attributes of other metals. Understanding this distinction between pure gold and its alloys is crucial for grasping the scientific classification of gold and its diverse applications.
Latest Posts
Latest Posts
-
Why Is The Earth Called Blue Planet
Mar 14, 2025
-
Least Common Multiple Of 21 And 28
Mar 14, 2025
-
How Many Valence Shell Electrons Does The Element Carbon Have
Mar 14, 2025
-
How Many Bones A Shark Have
Mar 14, 2025
-
In Situ Conservation Vs Ex Situ Conservation
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Is Gold A Mixture Or Pure Substance . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
