The Law Of Conservation Of Energy States That
Juapaving
Mar 05, 2025 · 6 min read
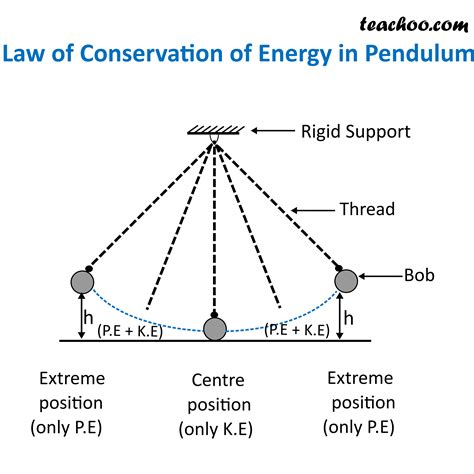
Table of Contents
The Law of Conservation of Energy: A Comprehensive Exploration
The Law of Conservation of Energy is a fundamental principle in physics, stating that energy cannot be created or destroyed, only transformed from one form to another. This seemingly simple statement underpins our understanding of the universe, from the smallest subatomic particles to the largest galaxies. Understanding this law is crucial across various scientific disciplines, impacting our daily lives in countless ways, from powering our homes to understanding the processes within our bodies. This article will delve deep into the law of conservation of energy, exploring its various facets, implications, and applications.
What Does the Law of Conservation of Energy Mean?
At its core, the law asserts that the total energy of an isolated system remains constant over time. An isolated system is one that doesn't exchange energy with its surroundings. This means that within this system, energy might change forms—from potential energy to kinetic energy, or from chemical energy to thermal energy—but the total amount remains the same.
Think of it like this: Imagine a ball rolling down a hill. Initially, the ball possesses potential energy due to its height. As it rolls, this potential energy is converted into kinetic energy (energy of motion). At the bottom of the hill, the potential energy is minimal, and the kinetic energy is maximal. However, ignoring friction and air resistance (ideal conditions for an isolated system), the total energy (potential + kinetic) remains unchanged throughout the process. The energy is simply transformed.
Different Forms of Energy
Energy exists in many forms, including:
- Kinetic Energy: The energy of motion. A moving car, a flying bird, even the molecules vibrating within a substance all possess kinetic energy.
- Potential Energy: Stored energy that has the potential to be converted into other forms of energy. Examples include gravitational potential energy (a ball held high above the ground), elastic potential energy (a stretched spring), and chemical potential energy (stored in bonds within molecules).
- Thermal Energy (Heat): The energy associated with the random motion of molecules. Higher temperatures indicate greater thermal energy.
- Radiant Energy (Light): Energy transmitted as electromagnetic waves, such as sunlight or light from a bulb.
- Electrical Energy: Energy carried by moving electric charges.
- Nuclear Energy: Energy stored within the nucleus of an atom, released through nuclear fission or fusion.
- Sound Energy: Energy transmitted through vibrations in a medium, like air or water.
The law of conservation of energy governs the transformations between all these forms. When one form of energy decreases, another form increases by an equivalent amount, maintaining the total energy constant.
Examples of the Law of Conservation of Energy in Action
The law of conservation of energy is observable in numerous everyday phenomena:
- A swinging pendulum: The pendulum's energy constantly changes between potential energy (at its highest points) and kinetic energy (at its lowest points). Ignoring energy losses due to friction and air resistance, the total energy remains constant throughout the swing.
- Hydroelectric power plants: These plants harness the potential energy of water stored in reservoirs behind dams. As the water flows downhill, its potential energy converts to kinetic energy, which then drives turbines to generate electricity.
- Photosynthesis: Plants convert radiant energy from sunlight into chemical potential energy stored in glucose molecules.
- Combustion: Burning fuel releases chemical potential energy in the form of heat and light.
- Rollercoasters: The coaster's potential energy at the top of a hill converts into kinetic energy as it descends, and this process continues throughout the ride, with energy losses primarily due to friction.
Limitations and Considerations
While the law of conservation of energy is a powerful and widely applicable principle, it does have some limitations and considerations:
- Non-isolated systems: The law applies strictly to isolated systems. In reality, it's difficult to achieve perfect isolation. Systems often exchange energy with their surroundings, meaning the total energy of the system might change.
- Energy losses due to friction and heat: In many real-world scenarios, energy is lost due to friction, air resistance, or other dissipative forces. This energy isn't destroyed; instead, it's transformed into thermal energy (heat), often dispersed into the environment, making it difficult to track the total energy within the system.
- Mass-energy equivalence: Einstein's famous equation, E=mc², shows that mass and energy are equivalent and interchangeable. This means that changes in mass can result in changes in energy, and vice-versa. This equivalence expands the scope of energy conservation to include mass as a form of energy.
The Law of Conservation of Energy and the Environment
The law of conservation of energy has significant implications for environmental issues:
- Energy efficiency: Understanding how energy transforms allows us to design more efficient systems and appliances, reducing energy waste and minimizing our environmental impact.
- Renewable energy sources: Harnessing renewable energy sources like solar, wind, and hydro power relies on the conversion of natural energy forms (solar radiation, wind kinetic energy, water potential energy) into usable electricity, all while adhering to the principle of conservation of energy.
- Climate change: The burning of fossil fuels releases significant amounts of energy, but this energy release also contributes to greenhouse gas emissions and climate change.
The Law of Conservation of Energy in Different Fields
The law's influence transcends physics, extending its reach into various fields:
- Engineering: Engineers apply the law to design efficient machines, power systems, and transportation systems.
- Chemistry: Chemical reactions involve the transformation of chemical potential energy into other forms, adhering to the principle of energy conservation.
- Biology: Biological processes, such as metabolism, are governed by the transformation and utilization of energy.
- Cosmology: Understanding the evolution of the universe requires considering the conservation of energy on a cosmic scale.
Beyond Conservation: The First Law of Thermodynamics
The law of conservation of energy is often referred to as the first law of thermodynamics. Thermodynamics deals with the relationships between heat, work, and energy. The first law states that the total energy of a system and its surroundings remains constant. This is a more comprehensive statement incorporating both the concept of energy conservation and the exchange of energy between a system and its environment.
Conclusion
The law of conservation of energy is a cornerstone of modern physics and has profound implications for our understanding of the universe and our place within it. While the concept seems simple at first glance, its ramifications are vast and far-reaching, influencing everything from the design of everyday appliances to the study of the cosmos. Understanding this law is critical not only for scientific advancement but also for addressing global challenges like climate change and energy sustainability. As we continue to explore the intricacies of the universe, the law of conservation of energy will remain a crucial guide, shaping our technological advancements and our comprehension of the fundamental laws that govern our world. It serves as a testament to the elegant simplicity and profound power of the fundamental principles that underpin the natural world.
Latest Posts
Latest Posts
-
Type Is A Grouping Based On Shared Characteristics
Mar 06, 2025
-
What Is The Sum Of Interior Angles In A Hexagon
Mar 06, 2025
-
Is Water A Renewable Or Nonrenewable Resource
Mar 06, 2025
-
What Are The Multiples Of 11
Mar 06, 2025
-
Which Of The Following Is An Intensive Property
Mar 06, 2025
Related Post
Thank you for visiting our website which covers about The Law Of Conservation Of Energy States That . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
