Which Of The Following Is An Intensive Property
Juapaving
Mar 06, 2025 · 6 min read
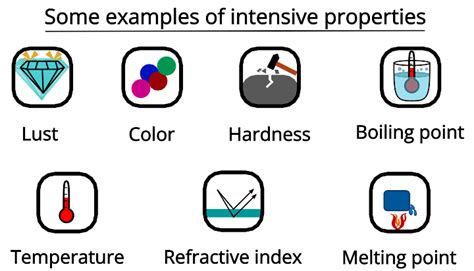
Table of Contents
Which of the Following is an Intensive Property? Understanding Intensive vs. Extensive Properties
Understanding the difference between intensive and extensive properties is crucial in various fields, from chemistry and physics to materials science and engineering. This comprehensive guide will delve into the definition, characteristics, and examples of intensive properties, contrasting them with extensive properties to provide a clear understanding of this fundamental concept. We'll also explore how this knowledge is applied in practical situations.
What are Intensive Properties?
Intensive properties are physical properties of a system that are independent of the system's size or amount of matter. This means that the value of an intensive property remains the same regardless of whether you have a small sample or a large quantity of the substance. In simpler terms, it's a property that describes the quality of a substance rather than its quantity.
Key characteristics of intensive properties:
- Independent of quantity: The value doesn't change with the amount of substance.
- Intrinsic to the material: It's a characteristic inherent to the material itself.
- Useful for identification: They are often used to identify and characterize substances.
Examples of Intensive Properties
Let's examine several common examples to solidify the concept:
1. Temperature
Temperature is a classic example. Whether you have a single drop of water or a swimming pool full of water, both will have the same temperature at a given point. The temperature doesn't change based on the amount of water present.
2. Density
Density, defined as mass per unit volume (mass/volume), is another excellent example. A small gold nugget and a large gold bar will have the same density, provided they are both made of pure gold. The density remains constant irrespective of the size or mass.
3. Pressure
Pressure exerted by a gas, for instance, is an intensive property. The pressure within a small balloon filled with air and a large inflated tire will be different, but for a given amount of gas in a defined volume, pressure is independent of the total amount of gas present.
4. Boiling Point
The boiling point of a substance is the temperature at which it changes from a liquid to a gas at a given pressure. This value is independent of the amount of substance; a small sample of water will boil at the same temperature as a large volume of water (at the same pressure).
5. Melting Point
Similar to boiling point, the melting point (or freezing point) is the temperature at which a solid changes to a liquid (or vice versa) at a given pressure. This is an intensive property because it remains constant regardless of the amount of substance.
6. Refractive Index
The refractive index measures how much light bends when passing through a substance. This is an inherent property of the material and is independent of the amount of material present.
7. Color
The color of a substance is an intensive property because it does not change with the amount of the substance. A small amount of blue dye will have the same color as a large amount of the same blue dye.
8. Hardness
Hardness, a measure of a substance's resistance to scratching or indentation, is another intensive property. A small diamond and a large diamond will both possess the same hardness.
9. Conductivity (Electrical and Thermal)
Electrical and thermal conductivity measure a material's ability to conduct electricity or heat, respectively. These properties are intensive; a small copper wire and a large copper bar will exhibit the same conductivity.
10. Specific Heat Capacity
Specific heat capacity is the amount of heat required to raise the temperature of one unit of mass of a substance by one degree Celsius (or Kelvin). This property is intensive, meaning that it remains constant irrespective of the mass of the substance.
What are Extensive Properties?
To fully grasp intensive properties, it's essential to contrast them with extensive properties. Extensive properties are physical properties that do depend on the amount of matter present. Their values change proportionally with the amount of substance.
Key characteristics of extensive properties:
- Dependent on quantity: The value changes with the amount of substance.
- Additive: The value for the whole system is the sum of the values for its parts.
Examples of Extensive Properties
Here are some common examples:
1. Mass
Mass is the amount of matter in an object. Clearly, a larger object has a greater mass than a smaller object.
2. Volume
Volume is the amount of space an object occupies. A larger object has a greater volume than a smaller object.
3. Length
The length of an object is directly proportional to its size.
4. Energy
The total energy of a system is dependent on the amount of matter.
5. Heat Capacity
The heat capacity of a substance is the amount of heat required to raise its temperature by one degree. This depends on the mass of the substance.
Differentiating Intensive and Extensive Properties: A Practical Approach
Consider a scenario where you have two beakers: one contains 100ml of water, and the other contains 500ml of water. Both are at room temperature (let's say 25°C).
- Intensive Property (Temperature): Both beakers have the same temperature (25°C), demonstrating that temperature is independent of the amount of water.
- Extensive Property (Volume): The volumes are different (100ml vs. 500ml), clearly showing that volume is dependent on the amount of water.
- Extensive Property (Mass): The mass of water in the larger beaker is greater than that in the smaller beaker.
The Importance of Understanding Intensive and Extensive Properties
The distinction between intensive and extensive properties is crucial in various scientific and engineering applications:
- Material characterization: Intensive properties are invaluable for identifying and characterizing materials, as they are intrinsic to the substance.
- Process optimization: Understanding intensive properties helps in optimizing various chemical and physical processes.
- Thermodynamics: Intensive properties play a crucial role in thermodynamic calculations and analyses.
- Phase diagrams: Intensive properties are used extensively in constructing and interpreting phase diagrams.
- Chemical engineering: Intensive properties are fundamental to various chemical engineering calculations and designs.
Addressing Common Confusions
Sometimes, the boundary between intensive and extensive properties can appear blurry. However, careful consideration usually clarifies the distinction. For example:
- Specific properties: Many extensive properties can be converted into intensive properties by dividing by mass or volume. For instance, heat capacity (extensive) becomes specific heat capacity (intensive) when divided by mass. This highlights that "specific" generally denotes an intensive property.
- Context matters: The context in which a property is discussed is crucial. Consider "concentration." While the total amount of solute in a solution is extensive, the concentration (amount of solute per unit volume) is intensive.
Conclusion
The distinction between intensive and extensive properties is a fundamental concept in science and engineering. Understanding these properties and their differences is critical for various applications, from characterizing materials to optimizing processes. By clearly understanding the characteristics and examples provided in this guide, you can confidently apply this knowledge to numerous scientific and engineering challenges. Remember that intensive properties are intrinsic to the material and independent of the amount of matter present, making them invaluable for identifying and characterizing substances. This understanding forms a strong foundation for further explorations in related scientific fields.
Latest Posts
Latest Posts
-
What Is The Molar Mass Of Butane C4h10
Mar 06, 2025
-
Function Of The Fine Adjustment Knob On A Microscope
Mar 06, 2025
-
Can Sugar Dissolve In Cold Water
Mar 06, 2025
-
Provide At Least Three Reasons Why Friction Is Needed
Mar 06, 2025
-
Can Acids Or Bases Conduct Electricity
Mar 06, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is An Intensive Property . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
