The Fundamental Unit Of Matter Is The
Juapaving
Mar 16, 2025 · 6 min read
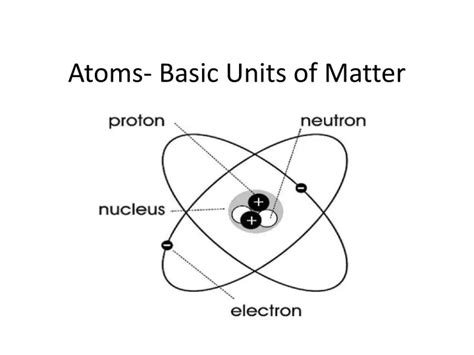
Table of Contents
The Fundamental Unit of Matter Is the Atom: A Deep Dive
The question, "What is the fundamental unit of matter?" has captivated scientists and philosophers for millennia. While the answer might seem simple – the atom – the reality is far more intricate and fascinating. This article delves deep into the atom, exploring its structure, properties, and its role in shaping the universe as we know it. We'll uncover its subatomic particles, delve into atomic models throughout history, and examine the implications of atomic theory in various scientific fields.
From Philosophers to Physicists: The Evolution of Atomic Theory
The concept of fundamental building blocks of matter isn't a recent invention. Ancient Greek philosophers like Leucippus and Democritus proposed the existence of atomos, indivisible particles, centuries before experimental evidence emerged. However, their ideas were largely philosophical speculations, lacking the rigorous scientific framework that would come much later.
Dalton's Atomic Theory: The First Scientific Model
John Dalton's work in the early 19th century marked a pivotal shift. His atomic theory, based on experimental observations, postulated that:
- All matter is composed of atoms: The fundamental building blocks of matter are indivisible and indestructible.
- Atoms of a given element are identical in mass and properties: Atoms of different elements have different masses and properties.
- Atoms of different elements can combine in simple whole-number ratios to form chemical compounds: This explained the law of definite proportions.
- Atoms cannot be created or destroyed in a chemical reaction: This explained the law of conservation of mass.
While Dalton's model was groundbreaking, it lacked an understanding of the internal structure of the atom. It considered the atom as a solid, indivisible sphere.
Thomson's Plum Pudding Model: Unveiling Subatomic Particles
J.J. Thomson's discovery of the electron in 1897 shattered the idea of the atom as an indivisible unit. His experiments with cathode rays revealed the existence of negatively charged particles far smaller than the atom itself. This led to the "plum pudding" model, where negatively charged electrons were embedded in a positively charged "pudding," maintaining overall neutrality.
Rutherford's Nuclear Model: The Atom's Central Core
Ernest Rutherford's gold foil experiment in 1911 revolutionized atomic theory once more. By bombarding a thin gold foil with alpha particles, he observed that most particles passed straight through, but some were deflected at large angles. This led to the revolutionary conclusion that the atom's positive charge and most of its mass were concentrated in a tiny, dense nucleus at its center, surrounded by a mostly empty space where electrons resided.
Bohr's Model: Quantized Energy Levels
Niels Bohr's model in 1913 incorporated the principles of quantum mechanics, addressing the limitations of Rutherford's model. Bohr proposed that electrons orbit the nucleus in specific energy levels or shells. Electrons can jump between these levels by absorbing or emitting energy in discrete amounts – a concept crucial to understanding atomic spectra.
The Quantum Mechanical Model: A Probabilistic View
The Bohr model, while a significant improvement, still had limitations. The current accepted model is the quantum mechanical model, a more sophisticated and probabilistic description of the atom. It utilizes wave functions to describe the probability of finding an electron at a particular location around the nucleus. This model acknowledges the inherent uncertainty in simultaneously knowing an electron's position and momentum (Heisenberg's uncertainty principle). Instead of fixed orbits, electrons occupy orbitals – regions of space where the probability of finding an electron is high.
Delving into the Subatomic Particles: The Building Blocks Within
The atom itself is not the ultimate fundamental unit; it's composed of even smaller constituents:
- Protons: Positively charged particles found in the nucleus. The number of protons defines an element's atomic number and its chemical identity.
- Neutrons: Neutral particles (no charge) also residing in the nucleus. Neutrons contribute to an atom's mass but not its charge. Isotopes of an element have the same number of protons but varying numbers of neutrons.
- Electrons: Negatively charged particles that orbit the nucleus in electron clouds or shells. The number of electrons generally equals the number of protons in a neutral atom.
Quarks and Leptons: A Deeper Dive into Fundamental Particles
Protons and neutrons are not fundamental themselves. They are composed of even smaller particles called quarks. There are six types (or "flavors") of quarks: up, down, charm, strange, top, and bottom. Protons consist of two up quarks and one down quark, while neutrons are made of one up quark and two down quarks.
Electrons, on the other hand, belong to a different class of fundamental particles called leptons. Leptons, unlike quarks, are not subject to the strong nuclear force. The electron is the most stable and common lepton.
Isotopes and Atomic Mass: Variations Within Elements
Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. This results in variations in atomic mass. For example, carbon-12 (¹²C) has six protons and six neutrons, while carbon-14 (¹⁴C) has six protons and eight neutrons. The atomic mass of an element is the weighted average of the masses of its isotopes, taking into account their relative abundance in nature.
The Role of Atomic Theory in Various Scientific Fields
Atomic theory underpins numerous scientific disciplines:
Chemistry: The foundation of chemical reactions, bonding, and the periodic table. Understanding atomic structure is crucial for predicting and explaining chemical behavior.
Nuclear Physics: The study of atomic nuclei, radioactivity, nuclear reactions, and applications like nuclear energy and medical imaging.
Material Science: The atomic structure of materials dictates their properties, allowing scientists to design materials with specific characteristics for various applications.
Astrophysics: Understanding the composition of stars and the processes occurring within them relies heavily on atomic theory. Spectral analysis of starlight reveals the elemental composition of celestial objects.
Biochemistry: The atoms that make up biological molecules, from DNA to proteins, dictate their functions and interactions within living organisms.
Nanotechnology: Manipulating individual atoms and molecules to create new materials and devices with novel properties.
The Ongoing Quest: Exploring the Frontiers of Atomic Physics
Despite our extensive knowledge of atomic structure, the quest to understand matter at its most fundamental level continues. Research in areas like particle physics delves into the properties of quarks, leptons, and other subatomic particles, seeking to unravel the mysteries of the universe's building blocks. The Standard Model of particle physics provides a comprehensive framework, but there are still open questions and ongoing efforts to develop more complete and accurate theories.
Conclusion: The Atom – A Foundation of Understanding
The atom, while not the ultimate fundamental particle, remains a cornerstone of our understanding of matter. From the philosophical musings of ancient Greece to the sophisticated quantum mechanical model of today, our journey to comprehend the atom has been a testament to human ingenuity and the power of scientific inquiry. Understanding the atom is not merely an academic pursuit; it is the foundation upon which many scientific and technological advancements are built, continuing to shape our world and drive future discoveries. The journey continues, with new questions and even deeper levels of understanding yet to be unveiled, enriching our appreciation for the intricate beauty and fundamental nature of the universe.
Latest Posts
Latest Posts
-
Function Of The Motor End Plate
Mar 17, 2025
-
A Push Or A Pull Is Called
Mar 17, 2025
-
The Tendency Of An Atom To Attract Electrons
Mar 17, 2025
-
What Are The Most Reactive Elements On The Periodic Table
Mar 17, 2025
-
Colorless Gas With A Pungent Odor
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about The Fundamental Unit Of Matter Is The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
