The Tendency Of An Atom To Attract Electrons
Juapaving
Mar 17, 2025 · 6 min read
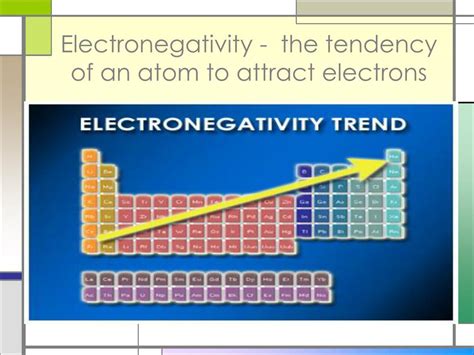
Table of Contents
The Tendency of an Atom to Attract Electrons: Electronegativity and its Implications
The universe is a dance of electrons. These subatomic particles, zipping around atomic nuclei, dictate the properties of matter, driving chemical reactions and shaping the world around us. Central to this dance is the tendency of an atom to attract electrons, a fundamental property known as electronegativity. Understanding electronegativity is key to unraveling the complexities of chemical bonding, molecular polarity, and the reactivity of elements. This article delves deep into this crucial concept, exploring its definition, trends across the periodic table, and its far-reaching consequences in chemistry and beyond.
What is Electronegativity?
Electronegativity quantifies an atom's ability to attract electrons towards itself within a chemical bond. It's not an absolute property; instead, it's a relative measure, comparing the electron-attracting power of one atom to another within a molecule. A higher electronegativity value indicates a stronger pull on shared electrons. Think of it like a tug-of-war: the atom with higher electronegativity pulls the electrons closer to its nucleus.
Key characteristics of electronegativity:
- Relative, not absolute: It's a comparison, not an inherent property of an isolated atom.
- Bond-dependent: Electronegativity varies slightly depending on the type of bond formed (e.g., single, double, triple).
- Context-specific: The value can change slightly based on the neighboring atoms in a molecule.
Several scales exist to represent electronegativity, the most common being the Pauling scale, developed by Linus Pauling. This scale assigns fluorine, the most electronegative element, a value of 4.0. Other elements are then assigned values relative to fluorine.
Trends in Electronegativity Across the Periodic Table
Electronegativity exhibits predictable trends across the periodic table, allowing us to estimate the relative electron-attracting power of different elements without extensive calculations. These trends are primarily governed by two factors:
1. Effective Nuclear Charge:
The effective nuclear charge (Z<sub>eff</sub>) is the net positive charge experienced by an electron in an atom. It's the actual positive charge felt by the electron after accounting for the shielding effect of inner electrons. As we move across a period (from left to right), the number of protons increases, increasing Z<sub>eff</sub>. This stronger positive charge pulls the valence electrons closer, increasing electronegativity.
2. Atomic Radius:
Atomic radius refers to the average distance between the nucleus and the outermost electrons. As we move across a period, the atomic radius decreases due to the increased nuclear charge. This closer proximity between the nucleus and valence electrons strengthens the attractive force, enhancing electronegativity. Conversely, moving down a group increases the atomic radius, reducing electronegativity as the valence electrons are further from the nucleus.
Summary of trends:
- Across a period (left to right): Electronegativity generally increases.
- Down a group (top to bottom): Electronegativity generally decreases.
The Impact of Electronegativity on Chemical Bonding
Electronegativity plays a pivotal role in determining the nature of chemical bonds. The difference in electronegativity between two atoms dictates whether the bond is nonpolar covalent, polar covalent, or ionic.
1. Nonpolar Covalent Bonds:
When two atoms with similar electronegativities bond, the electrons are shared relatively equally between them. This results in a nonpolar covalent bond, where there's no significant charge separation. Examples include bonds between two identical atoms (e.g., H<sub>2</sub>, O<sub>2</sub>) or atoms with very small electronegativity differences.
2. Polar Covalent Bonds:
When atoms with significantly different electronegativities bond, the more electronegative atom pulls the shared electrons closer to itself. This creates a partial negative charge (δ-) on the more electronegative atom and a partial positive charge (δ+) on the less electronegative atom. This unequal sharing of electrons results in a polar covalent bond, creating a dipole moment – a separation of charge within the molecule. Water (H<sub>2</sub>O) is a classic example, with oxygen being more electronegative than hydrogen.
3. Ionic Bonds:
In extreme cases, where the electronegativity difference between two atoms is very large, the more electronegative atom essentially steals the electron from the less electronegative atom. This results in the formation of ions: a positively charged cation (from the atom that lost the electron) and a negatively charged anion (from the atom that gained the electron). These oppositely charged ions are then attracted to each other through electrostatic forces, forming an ionic bond. Sodium chloride (NaCl) is a prime example, with chlorine having a much higher electronegativity than sodium.
Electronegativity and Molecular Properties
The electronegativity of atoms within a molecule significantly influences its overall properties:
1. Molecular Polarity:
The presence of polar bonds doesn't automatically mean the entire molecule is polar. Molecular geometry plays a crucial role. If the polar bonds are symmetrically arranged, their dipole moments can cancel each other out, resulting in a nonpolar molecule (e.g., carbon dioxide, CO<sub>2</sub>). However, if the polar bonds are arranged asymmetrically, the dipole moments will add up, resulting in a polar molecule (e.g., water, H<sub>2</sub>O).
2. Boiling Point and Melting Point:
Polar molecules have stronger intermolecular forces (dipole-dipole interactions and hydrogen bonding) compared to nonpolar molecules (only weak London dispersion forces). This leads to higher boiling and melting points for polar molecules.
3. Solubility:
"Like dissolves like" is a fundamental principle in chemistry. Polar solvents tend to dissolve polar molecules, while nonpolar solvents dissolve nonpolar molecules. This is because similar intermolecular forces facilitate interaction and dissolution.
4. Reactivity:
Electronegativity differences influence a molecule's reactivity. Polar molecules often react more readily than nonpolar molecules due to the presence of partial charges, creating sites for electrophilic and nucleophilic attacks.
Electronegativity and Beyond: Applications and Importance
Understanding electronegativity is essential in various fields:
- Chemistry: Predicting the type of bond formed, molecular geometry, polarity, reactivity, and physical properties of compounds.
- Materials Science: Designing materials with specific properties by tailoring the electronegativity of constituent atoms.
- Biochemistry: Understanding the interactions between biomolecules, such as protein folding and enzyme-substrate interactions, where electronegativity plays a significant role.
- Drug Discovery: Designing drugs with specific properties by manipulating the electronegativity of functional groups.
Conclusion
The tendency of an atom to attract electrons, quantified by electronegativity, is a fundamental concept underpinning much of chemistry. Its impact extends far beyond simple bonding descriptions, influencing molecular polarity, reactivity, and a wide range of physical and chemical properties. Understanding the trends and implications of electronegativity is crucial for anyone studying chemistry, materials science, biochemistry, or related fields. By grasping this concept, we can better understand the intricate dance of electrons that shapes our world. Further research continues to refine our understanding of electronegativity and its subtle nuances, contributing to advancements in various scientific disciplines. The seemingly simple attraction of electrons to an atom's nucleus unlocks a universe of complex and fascinating phenomena.
Latest Posts
Latest Posts
-
Food Is Digested Physical Or Chemical Change
Mar 17, 2025
-
What Are The Factor Pairs Of 56
Mar 17, 2025
-
Are Lysosomes Only In Animal Cells
Mar 17, 2025
-
What Are The Prime Factors Of 47
Mar 17, 2025
-
Electronic Configuration Of Cr And Cu
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about The Tendency Of An Atom To Attract Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
