The Atomic Number Is Equal To The
Juapaving
Mar 25, 2025 · 6 min read
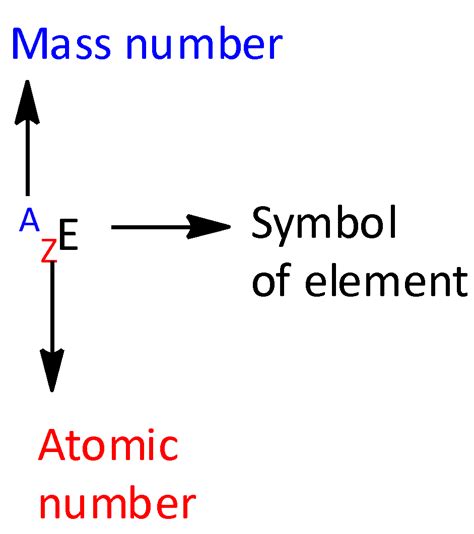
Table of Contents
The Atomic Number is Equal To the Number of Protons: A Deep Dive into Atomic Structure
The seemingly simple statement, "the atomic number is equal to the number of protons," underpins our entire understanding of chemistry and the periodic table. This seemingly basic fact is the cornerstone upon which countless scientific discoveries and technological advancements have been built. Let's delve into the intricacies of atomic structure, exploring what atomic number truly represents, its significance in determining an element's properties, and its crucial role in various scientific disciplines.
Understanding Atomic Structure: A Foundation for Chemistry
Before we solidify the relationship between atomic number and protons, let's first establish a firm grasp on the fundamental building blocks of matter: atoms. Atoms are the smallest units of an element that retain the chemical properties of that element. They are composed of three primary subatomic particles:
1. Protons: Positively Charged Core
Protons reside within the atom's nucleus, the dense central region. They carry a positive electrical charge (+1) and possess a mass approximately 1836 times greater than that of an electron. The number of protons in an atom's nucleus is what uniquely defines an element. This is where the crucial connection to the atomic number comes into play.
2. Neutrons: Neutral Nuclear Partners
Neutrons, also located in the nucleus, are electrically neutral; they carry no charge. Their mass is very slightly greater than that of a proton. The number of neutrons in an atom's nucleus can vary, leading to different isotopes of the same element. Isotopes are atoms of the same element with the same number of protons but a different number of neutrons.
3. Electrons: Negatively Charged Orbitals
Electrons are negatively charged particles (-1) that orbit the nucleus in specific energy levels or shells. Their mass is significantly less than that of protons and neutrons. The arrangement of electrons in these shells determines an element's chemical behavior and how it interacts with other atoms. The number of electrons in a neutral atom is equal to the number of protons.
Atomic Number: The Defining Characteristic of an Element
The atomic number is a fundamental property of an element. It represents the number of protons found in the nucleus of an atom of that element. This number is unique to each element and is represented by the symbol Z. For example:
- Hydrogen (H): Atomic number Z = 1 (1 proton)
- Helium (He): Atomic number Z = 2 (2 protons)
- Oxygen (O): Atomic number Z = 8 (8 protons)
- Gold (Au): Atomic number Z = 79 (79 protons)
The atomic number is crucial because it:
- Uniquely identifies an element: No two elements share the same atomic number. This is what allows us to distinguish between different elements on the periodic table.
- Determines the chemical properties: The number of protons dictates the number of electrons in a neutral atom, which in turn determines the atom's electronic configuration and its chemical behavior. Elements with similar electronic configurations exhibit similar chemical properties, as seen in the periodic table's arrangement.
- Predicts the number of electrons (in neutral atoms): In a neutral atom, the number of electrons equals the number of protons. This is because the positive charge of the protons is balanced by the negative charge of the electrons.
- Forms the basis of the periodic table: The periodic table is organized by increasing atomic number, showcasing the periodic trends in elemental properties.
Isotopes: Variations in Neutron Number
While the atomic number remains constant for a given element, the number of neutrons can vary. These variations give rise to isotopes. Isotopes of an element have the same atomic number (same number of protons) but a different mass number (the sum of protons and neutrons).
For example, carbon (atomic number 6) has three naturally occurring isotopes:
- Carbon-12 (¹²C): 6 protons, 6 neutrons
- Carbon-13 (¹³C): 6 protons, 7 neutrons
- Carbon-14 (¹⁴C): 6 protons, 8 neutrons
All three are carbon because they all have 6 protons. However, they differ in their mass due to the varying number of neutrons. This difference in neutron number can affect the stability of the isotope; some isotopes are radioactive, meaning they decay over time, emitting radiation. Carbon-14, for example, is a radioactive isotope used in radiocarbon dating.
The Significance of Atomic Number in Chemistry and Beyond
The concept of atomic number is not merely an abstract scientific concept; it has far-reaching implications across various scientific fields and technological applications:
1. Chemistry: Understanding Chemical Reactions
The atomic number plays a pivotal role in understanding chemical reactions. The arrangement of electrons, determined by the number of protons (and therefore the atomic number), dictates how atoms interact with each other. This interaction determines the types of chemical bonds that form (ionic, covalent, metallic) and the resulting chemical properties of compounds.
2. Nuclear Physics: Nuclear Reactions and Radioactivity
Nuclear physics relies heavily on atomic number to understand nuclear reactions. Nuclear reactions involve changes in the nucleus of an atom, such as nuclear fission (splitting of a nucleus) and nuclear fusion (combining of nuclei). The atomic number changes during these reactions, resulting in the transformation of one element into another. Radioactivity, the spontaneous emission of radiation from unstable isotopes, is also directly related to the number of protons and neutrons in the nucleus.
3. Materials Science: Designing New Materials
Materials science uses knowledge of atomic numbers to design and create new materials with specific properties. By manipulating the composition and arrangement of atoms, scientists can tailor materials for specific applications, such as stronger alloys, more efficient semiconductors, and novel superconductors. Understanding how atomic number influences the interaction between atoms is crucial in this field.
4. Medical Applications: Medical Imaging and Treatment
The atomic number plays a vital role in medical applications, notably in medical imaging techniques like X-rays and CT scans. These techniques rely on the interaction of X-rays with the atoms in the body. The atomic number of different tissues and organs influences how they absorb and scatter X-rays, allowing for visualization of internal structures. Additionally, radioactive isotopes with specific atomic numbers are used in various medical treatments, such as radiation therapy for cancer.
5. Astrophysics and Cosmology: Understanding Stellar Nucleosynthesis
In astrophysics, the atomic number is crucial for understanding the processes that occur within stars, namely stellar nucleosynthesis. Stars create heavier elements through nuclear fusion, where lighter atomic nuclei combine to form heavier ones. The atomic number increases as heavier elements are synthesized, leading to the creation of all the elements heavier than hydrogen and helium. Understanding this process helps astronomers to comprehend the origin of elements in the universe and the evolution of stars.
Conclusion: The Fundamental Importance of Atomic Number
The statement "the atomic number is equal to the number of protons" is far more profound than it initially appears. This seemingly simple equation is the cornerstone of our understanding of the fundamental building blocks of matter and their interactions. It is a defining characteristic of an element, determining its chemical properties, its behavior in various reactions, and its role in countless scientific and technological applications. From understanding chemical reactions to designing new materials and exploring the cosmos, the atomic number remains a central concept across numerous scientific disciplines. Its importance continues to grow as scientists delve deeper into the intricacies of the atomic world. The continued study and application of this fundamental principle will undoubtedly lead to further breakthroughs and advancements in science and technology.
Latest Posts
Latest Posts
-
What Are The Prime Factors Of 15
Mar 27, 2025
-
9 Rounded To The Nearest Tenth
Mar 27, 2025
-
What Percent Is 15 Of 50
Mar 27, 2025
-
Nh4 3po4 Pb No3 4 Pb3 Po4 4 Nh4no3
Mar 27, 2025
-
In Which Figure Is Point G A Centroid
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about The Atomic Number Is Equal To The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
