Relationship Between Mass And Volume And Density
Juapaving
Mar 23, 2025 · 7 min read
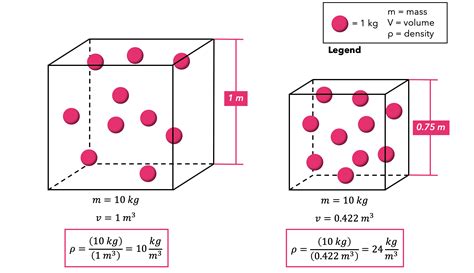
Table of Contents
The Intimate Relationship Between Mass, Volume, and Density
Understanding the relationship between mass, volume, and density is fundamental to physics and chemistry. These three properties are intrinsically linked, describing the fundamental characteristics of matter. While seemingly simple, mastering their relationship unlocks a deeper understanding of how substances behave and interact. This comprehensive guide explores the intricacies of mass, volume, and density, providing clear definitions, practical examples, and insights into their applications in various scientific fields.
Defining the Key Players: Mass, Volume, and Density
Before delving into their relationship, let's clearly define each term:
Mass: The Measure of Inertia
Mass is a scalar quantity representing the amount of matter in an object. It measures an object's resistance to acceleration, a property known as inertia. A more massive object requires a greater force to achieve the same acceleration as a less massive object. The standard unit of mass is the kilogram (kg), although grams (g) are also commonly used. It's crucial to distinguish mass from weight; weight is the force of gravity acting on an object's mass, and therefore varies depending on location (e.g., weight on the Moon is less than on Earth, while mass remains constant).
Volume: The Measure of Space Occupied
Volume is a scalar quantity representing the amount of three-dimensional space occupied by an object or substance. It's essentially the object's size. The standard unit of volume is the cubic meter (m³), but other units like liters (L), milliliters (mL), and cubic centimeters (cm³) are frequently employed, especially in chemistry and everyday life. Measuring volume can involve various techniques, depending on the object's shape and state (solid, liquid, or gas).
Density: Mass per Unit Volume
Density is a scalar quantity defined as the mass of a substance per unit volume. It essentially tells us how much matter is packed into a given space. A higher density means more mass is concentrated in a smaller volume. The standard unit of density is kilograms per cubic meter (kg/m³), but grams per cubic centimeter (g/cm³) is also widely used, particularly when dealing with solids and liquids. Density is a crucial property because it's unique to each substance under specific conditions (temperature and pressure).
The Mathematical Relationship: The Density Formula
The relationship between mass, volume, and density is expressed by a simple yet powerful formula:
Density (ρ) = Mass (m) / Volume (V)
This formula allows us to calculate any of the three properties if we know the other two. For instance:
- Calculating Density: If we know the mass and volume of an object, we can calculate its density using the formula directly.
- Calculating Mass: If we know the density and volume of a substance, we can rearrange the formula to calculate its mass: m = ρV
- Calculating Volume: Similarly, if we know the density and mass of a substance, we can rearrange the formula to calculate its volume: V = m/ρ
Factors Affecting Density
Density isn't a constant property; it can be affected by various factors:
Temperature: Thermal Expansion and Contraction
Temperature significantly impacts density. Generally, as temperature increases, the volume of a substance expands (thermal expansion), leading to a decrease in density. Conversely, as temperature decreases, the volume contracts (thermal contraction), resulting in an increase in density. This is why hot air rises (less dense) and cold air sinks (more dense). The exception is water, which exhibits unusual behavior near its freezing point.
Pressure: Compressibility
Pressure also influences density, especially in gases and liquids. Increasing pressure compresses a substance, reducing its volume and thereby increasing its density. Solids are generally less compressible and exhibit a smaller change in density under pressure.
Composition: Mixtures and Solutions
The composition of a substance directly affects its density. Mixtures and solutions have densities that depend on the densities and proportions of their components. For instance, saltwater is denser than freshwater because the dissolved salt adds mass without significantly increasing volume.
Applications of Density: Real-World Examples
Understanding and utilizing density is crucial in numerous applications across various scientific and engineering disciplines:
Material Science and Engineering: Selecting Appropriate Materials
Engineers use density to select appropriate materials for specific applications. For example, lightweight yet strong materials with low densities are preferred for aerospace applications, while high-density materials are suitable for shielding against radiation. Density considerations also play a critical role in structural design and construction.
Geology and Geophysics: Determining Earth's Structure
Geophysicists use density measurements to study the Earth's internal structure. Variations in density at different depths provide insights into the composition and physical properties of the Earth's layers (crust, mantle, core). Density data from seismic waves helps create models of the Earth's interior.
Oceanography: Studying Ocean Currents and Water Masses
Oceanographers use density to study ocean currents and water masses. Differences in salinity and temperature create density variations, which drive ocean circulation patterns. Understanding these density gradients is essential for predicting climate change effects and marine ecosystems.
Meteorology: Understanding Atmospheric Phenomena
Meteorologists use density measurements to understand atmospheric phenomena like weather patterns and air circulation. Variations in air density due to temperature and pressure differences contribute to wind formation and the movement of weather systems.
Medicine and Healthcare: Diagnosing Diseases
Density measurements are employed in medical imaging techniques like X-rays and computed tomography (CT) scans. Different tissues and organs have varying densities, allowing doctors to visualize internal structures and identify abnormalities. Bone density measurements are also crucial in diagnosing osteoporosis.
Density and Buoyancy: Archimedes' Principle
Density plays a critical role in buoyancy, the ability of an object to float in a fluid (liquid or gas). Archimedes' principle states that an object immersed in a fluid experiences an upward buoyant force equal to the weight of the fluid displaced by the object. Whether an object floats or sinks depends on the comparison between its density and the density of the fluid:
- Object denser than the fluid: The buoyant force is less than the object's weight, causing it to sink.
- Object less dense than the fluid: The buoyant force is greater than the object's weight, allowing it to float.
- Object with the same density as the fluid: The buoyant force equals the object's weight, causing it to remain suspended in the fluid.
Advanced Concepts: Relative Density and Specific Gravity
While density provides an absolute measure of mass per unit volume, sometimes a relative measure is more useful. This leads to the concepts of relative density and specific gravity:
Relative Density: Comparing Densities
Relative density compares the density of a substance to the density of a reference substance, usually water at 4°C (its maximum density). It's a dimensionless quantity, calculated as:
Relative Density = Density of substance / Density of water
Specific Gravity: Another Name for Relative Density
Specific gravity is essentially another term for relative density. It represents the ratio of the density of a substance to the density of water at a specified temperature (usually 4°C). It's often used in various industries, like the petroleum industry, to characterize the density of different liquids.
Conclusion: The Significance of Mass, Volume, and Density
The relationship between mass, volume, and density is fundamental to our understanding of matter and its behavior. From everyday applications like cooking and baking (measuring ingredients) to complex scientific investigations like studying the Earth's interior or designing spacecraft, the concepts of mass, volume, and density are indispensable. Mastering these concepts opens doors to a deeper appreciation of the physical world and its intricate workings. The simple formula connecting these three properties serves as a cornerstone of numerous scientific disciplines, emphasizing the power of basic principles in unlocking a deeper understanding of the universe around us. Further exploration of these principles leads to a more profound appreciation of the scientific method and the interconnectedness of seemingly disparate phenomena.
Latest Posts
Latest Posts
-
What Is The Difference Between Exothermic And Endothermic
Mar 25, 2025
-
Diagram Of An Animal Cell With Labels
Mar 25, 2025
-
One To One Function And Inverse Function
Mar 25, 2025
-
When Does Segregation Of Alleles Occur
Mar 25, 2025
-
How Many 1 5 Liters In A Gallon
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Relationship Between Mass And Volume And Density . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
