What Is The Difference Between Exothermic And Endothermic
Juapaving
Mar 25, 2025 · 6 min read
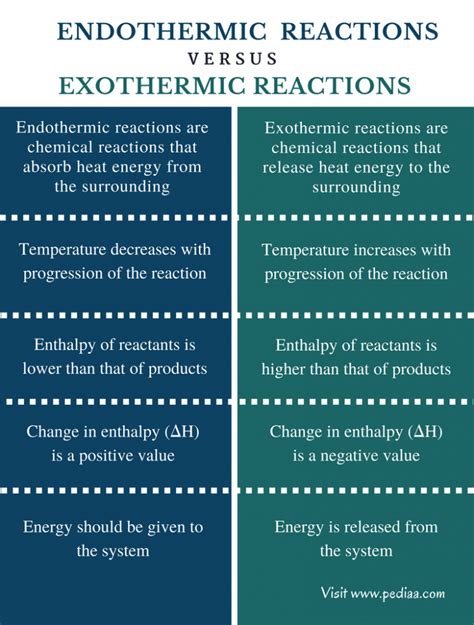
Table of Contents
What's the Difference Between Exothermic and Endothermic Reactions?
Understanding the difference between exothermic and endothermic reactions is fundamental to grasping many concepts in chemistry and physics. These terms describe the energy transfer that occurs during a chemical or physical process, influencing everything from everyday occurrences like combustion to complex industrial processes. This comprehensive guide will delve deep into the intricacies of exothermic and endothermic reactions, exploring their definitions, characteristics, examples, and applications.
Defining Exothermic and Endothermic Reactions
At the heart of the distinction lies the concept of energy transfer. Chemical reactions involve the breaking and forming of chemical bonds. Breaking bonds requires energy input, while forming bonds releases energy. Whether a reaction is exothermic or endothermic depends on the net energy change – the difference between the energy required to break bonds and the energy released when new bonds are formed.
Exothermic reactions are characterized by the release of energy into the surroundings. This energy is often manifested as heat, resulting in an increase in the temperature of the system's surroundings. The enthalpy change (ΔH) for an exothermic reaction is negative, indicating a decrease in the system's enthalpy (heat content). Think of it like this: the system is losing energy, and that energy is transferred to the surroundings.
Endothermic reactions, on the other hand, absorb energy from their surroundings. This energy absorption causes a decrease in the temperature of the surroundings. The enthalpy change (ΔH) for an endothermic reaction is positive, indicating an increase in the system's enthalpy. The system is gaining energy from its environment.
Key Differences Summarized
| Feature | Exothermic Reaction | Endothermic Reaction |
|---|---|---|
| Energy Transfer | Releases energy to the surroundings | Absorbs energy from the surroundings |
| Enthalpy Change (ΔH) | Negative (ΔH < 0) | Positive (ΔH > 0) |
| Temperature Change | Surroundings get warmer | Surroundings get colder |
| Bond Energy | Energy released from bond formation > energy needed to break bonds | Energy needed to break bonds > energy released from bond formation |
| Examples | Combustion, neutralization reactions, condensation | Photosynthesis, melting ice, boiling water |
Exothermic Reactions: A Closer Look
Exothermic reactions are prevalent in our daily lives and industrial processes. They are often associated with the release of heat, light, or sound. Let's explore some key characteristics and examples:
Characteristics of Exothermic Reactions:
- Heat release: The most prominent characteristic is the production of heat, making the surroundings warmer.
- Negative enthalpy change: The system's enthalpy decreases as energy is released.
- Spontaneous nature (often): Many exothermic reactions occur spontaneously, although this is not always the case. Spontaneity depends on both enthalpy and entropy changes.
- Applications in various fields: From generating electricity in power plants to powering everyday appliances.
Examples of Exothermic Reactions:
- Combustion: The burning of fuels like wood, propane, or gasoline is a classic example. The chemical energy stored in the fuel is converted into heat and light.
- Neutralization Reactions: The reaction between an acid and a base produces water and heat. For example, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) releases a significant amount of heat.
- Respiration: The process by which living organisms release energy from food is exothermic. Cellular respiration breaks down glucose to produce ATP, releasing energy in the form of heat.
- Condensation: When a gas changes into a liquid (like water vapor forming dew), it releases energy in the form of heat.
- Nuclear fission: The splitting of heavy atomic nuclei, such as uranium, releases enormous amounts of energy as heat.
Endothermic Reactions: A Detailed Examination
Endothermic reactions, while perhaps less immediately apparent in everyday life, are equally important and are fundamental to many natural and industrial processes.
Characteristics of Endothermic Reactions:
- Heat absorption: The key feature is the absorption of heat from the surroundings, resulting in a temperature decrease.
- Positive enthalpy change: The system's enthalpy increases as it absorbs energy.
- Often non-spontaneous: Many endothermic reactions require energy input to proceed.
- Important in various biological and industrial processes: Essential for processes like photosynthesis and industrial productions requiring high temperatures.
Examples of Endothermic Reactions:
- Photosynthesis: Plants absorb sunlight to convert carbon dioxide and water into glucose and oxygen. This process requires energy input from the sun.
- Melting ice: Converting ice into liquid water requires energy input to break the hydrogen bonds holding the water molecules in a solid state.
- Boiling water: Similar to melting, converting liquid water into steam necessitates energy input to overcome the intermolecular forces.
- Dissolving ammonium nitrate: Dissolving ammonium nitrate in water absorbs heat, leading to a noticeable temperature decrease.
- Electrolysis: The decomposition of water into hydrogen and oxygen using electricity requires energy input.
- Cooking an egg: Cooking an egg is an example of a complex endothermic process, needing heat input to denature the proteins.
Enthalpy Change (ΔH) and its Significance
The enthalpy change (ΔH) is a crucial parameter used to quantify the heat transferred during a chemical reaction at constant pressure. It represents the difference between the enthalpy of the products and the enthalpy of the reactants.
- ΔH < 0 (Negative): Exothermic reaction; energy is released.
- ΔH > 0 (Positive): Endothermic reaction; energy is absorbed.
The magnitude of ΔH indicates the amount of heat transferred. A larger value signifies a greater energy change.
Factors Affecting Exothermic and Endothermic Reactions
Several factors influence the rate and extent of both exothermic and endothermic reactions. These include:
- Temperature: Increasing temperature generally increases the rate of both exothermic and endothermic reactions. However, the effect on the equilibrium position differs; in endothermic reactions, increasing temperature shifts the equilibrium to favor products. In exothermic reactions, the opposite is true.
- Concentration: Higher reactant concentrations usually lead to faster reaction rates for both types of reactions.
- Surface area: Increasing the surface area of solid reactants accelerates reaction rates, particularly in heterogeneous reactions.
- Catalyst: Catalysts increase the rate of reaction without being consumed themselves; they do this by lowering the activation energy, accelerating both exothermic and endothermic reactions.
- Pressure: Pressure changes significantly affect the rates and equilibrium positions of reactions involving gases.
Applications of Exothermic and Endothermic Reactions
The principles of exothermic and endothermic reactions are exploited across diverse fields:
Exothermic Reactions Applications:
- Power Generation: Combustion of fossil fuels in power plants to generate electricity.
- Heating: Natural gas combustion for heating homes and buildings.
- Industrial Processes: Many industrial processes, such as cement production and steelmaking, rely on exothermic reactions.
- Welding: Exothermic reactions provide the heat needed for welding metals.
Endothermic Reactions Applications:
- Refrigeration: Endothermic processes are crucial in refrigeration systems, absorbing heat from the surroundings to cool them.
- Industrial Processes: Some industrial processes, such as ammonia production (Haber-Bosch process), are endothermic and require significant energy input.
- Chemical Synthesis: Many chemical syntheses rely on endothermic reactions to produce desired products.
- Medical Applications: Some endothermic processes are employed in medical applications, such as the use of cold packs for injury treatment.
Conclusion
The distinction between exothermic and endothermic reactions hinges on the direction of energy flow. Exothermic reactions release energy, often as heat, while endothermic reactions absorb energy from their surroundings. Understanding these fundamental concepts is crucial for comprehending numerous chemical and physical phenomena, from everyday processes to sophisticated technological applications. This knowledge underpins advancements in various fields, showcasing the pervasive importance of energy transfer in the natural world and human endeavors. By recognizing the differences and applications of these reactions, we can better understand and harness the power of energy transformations.
Latest Posts
Latest Posts
-
Label The Cross Section Of A Leaf
Mar 26, 2025
-
Difference Between Plant Mitosis And Animal Mitosis
Mar 26, 2025
-
How Many Energy Levels Does Potassium Have
Mar 26, 2025
-
5 Letter Word Ending In Ing
Mar 26, 2025
-
How Many Feet In 33 Inches
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between Exothermic And Endothermic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
