Number Of Valence Electrons In Sr
Juapaving
Mar 23, 2025 · 5 min read
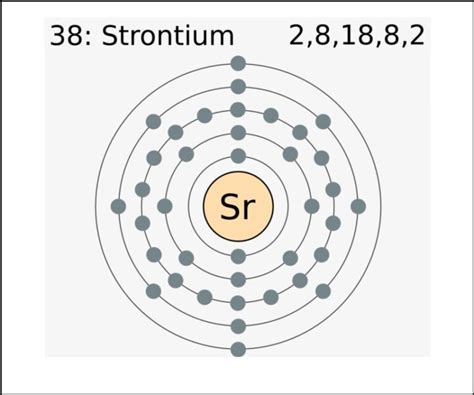
Table of Contents
Unveiling the Valence Electrons of Strontium: A Deep Dive into Atomic Structure and Chemical Behavior
Strontium (Sr), a silvery-white alkaline earth metal, plays a fascinating role in various scientific and technological applications. Understanding its chemical behavior is crucial, and this hinges on knowing its number of valence electrons. This article delves deep into the electronic configuration of strontium, explaining why it possesses the number of valence electrons it does, and exploring the implications this has for its reactivity and bonding characteristics. We'll also touch on its applications and explore related concepts like ionization energy and electronegativity.
Understanding Valence Electrons: The Key to Reactivity
Before focusing on strontium, let's clarify the concept of valence electrons. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are crucial because they determine an element's chemical properties and how it interacts with other atoms to form chemical bonds. They are the primary players in chemical reactions, dictating an element's ability to gain, lose, or share electrons to achieve a stable electron configuration, typically resembling that of a noble gas.
The Significance of the Octet Rule
The octet rule, a fundamental principle in chemistry, states that atoms tend to gain, lose, or share electrons to achieve a full outer electron shell containing eight electrons (or two electrons for the first shell, as in helium). This stable configuration minimizes the atom's energy and enhances its stability. Elements achieve this stability through chemical bonding, where they interact with other atoms to share or transfer valence electrons.
Determining the Number of Valence Electrons in Strontium (Sr)
Strontium's atomic number is 38, meaning it possesses 38 protons and 38 electrons in a neutral atom. To determine the number of valence electrons, we need to examine its electron configuration. This configuration describes how electrons are distributed among the different energy levels or shells within the atom.
Strontium's Electron Configuration
The electron configuration of strontium is [Kr]5s². Let's break this down:
-
[Kr]: This represents the electron configuration of krypton (Kr), a noble gas with atomic number 36. We use this shorthand notation because the inner electrons of strontium are arranged identically to those of krypton. These inner electrons are relatively inert and don't typically participate in chemical bonding.
-
5s²: This indicates that strontium has two electrons in the 5s subshell, which is the outermost electron shell.
Therefore, strontium has two valence electrons. These two electrons in the 5s orbital are readily available to participate in chemical bonding.
Implications of Strontium's Two Valence Electrons
The presence of two valence electrons significantly influences strontium's chemical behavior:
1. Reactivity:
Strontium readily loses its two valence electrons to achieve a stable electron configuration like that of krypton. This makes it a highly reactive metal, particularly with nonmetals such as oxygen, chlorine, and halogens. The loss of these electrons forms a +2 cation (Sr²⁺).
2. Bonding:
Because strontium readily loses two electrons, it primarily forms ionic bonds. Ionic bonding involves the electrostatic attraction between positively charged cations (like Sr²⁺) and negatively charged anions (like Cl⁻ or O²⁻). Examples include strontium chloride (SrCl₂) and strontium oxide (SrO).
3. Oxidation State:
Strontium's most common oxidation state is +2, reflecting its tendency to lose two electrons during chemical reactions.
Strontium's Ionization Energy
Ionization energy is the energy required to remove an electron from a gaseous atom or ion. Strontium's first ionization energy (the energy required to remove the first electron) is relatively low compared to other elements, consistent with its tendency to lose electrons easily. The second ionization energy (removing the second electron) is higher, but still relatively manageable, further illustrating its preference for a +2 oxidation state. Subsequent ionization energies would be significantly higher, as they would involve removing electrons from the stable, inner electron shells.
Electronegativity of Strontium
Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. Strontium possesses a low electronegativity. This signifies its weaker ability to attract electrons compared to other elements, reinforcing its tendency to lose electrons instead of gaining them. This low electronegativity further emphasizes its preference for ionic bonding with more electronegative elements.
Applications of Strontium and its Compounds
Strontium's unique properties, stemming from its electronic configuration and reactivity, lead to various applications:
1. Pyrotechnics:
Strontium salts, particularly strontium carbonate (SrCO₃) and strontium nitrate (Sr(NO₃)₂), are extensively used in fireworks to produce a brilliant red color. The excited strontium ions emit red light when they return to their ground state after being energized by the heat of the combustion process. The intensity of the red color makes strontium compounds invaluable in creating visually stunning pyrotechnic displays.
2. Medical Applications:
Strontium ranelate, a strontium compound, has been used in the treatment of osteoporosis, a condition characterized by weakened bones. It acts by stimulating bone formation and reducing bone resorption.
3. Production of Alloys:
Strontium is sometimes added to aluminum and magnesium alloys to improve their mechanical properties, such as strength and castability. These alloys are used in various applications where these enhanced properties are critical.
4. Nuclear Applications:
Strontium-90, a radioactive isotope of strontium, is a significant byproduct of nuclear fission. Although hazardous, it also finds limited applications in certain specialized industrial gauges and in radioisotope thermoelectric generators. The handling of radioactive strontium requires rigorous safety precautions.
5. Other Uses:
Strontium is also employed in various other niche applications, including in the production of certain types of glass, in catalysts, and in some specialty ceramics.
Conclusion: Strontium's Valence Electrons Shape its World
The two valence electrons of strontium are the key to understanding its chemical and physical behavior. They dictate its reactivity, bonding preferences, and ultimately, its diverse applications in various fields. Its tendency to readily lose these electrons to form a +2 cation drives its participation in ionic compounds and its use in pyrotechnics, alloys, and even medical applications. The consistent loss of these two electrons underlines its characteristic chemical behavior and its overall importance across a range of scientific and technological domains. Further research continues to explore new applications and refine our understanding of this reactive and versatile alkaline earth metal. Understanding the fundamental aspects of atomic structure, such as the number of valence electrons, remains a cornerstone in comprehending the rich complexity of the chemical world and its boundless applications.
Latest Posts
Latest Posts
-
Light Microscope And Electron Microscope Differences
Mar 25, 2025
-
A Catalyst Lowers The Activation Energy Of A Reaction By
Mar 25, 2025
-
Whats The Lcm Of 8 And 10
Mar 25, 2025
-
What Is 11 Cm In Inches
Mar 25, 2025
-
Least Common Multiple Of 40 And 15
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons In Sr . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
