A Catalyst Lowers The Activation Energy Of A Reaction By
Juapaving
Mar 25, 2025 · 6 min read
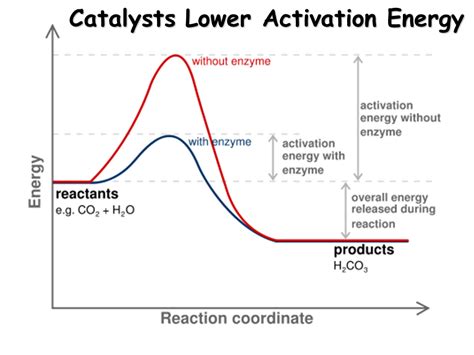
Table of Contents
A Catalyst Lowers the Activation Energy of a Reaction By… Altering the Reaction Pathway
Chemical reactions are the fundamental processes that govern all aspects of the natural world, from the growth of a plant to the burning of fuel. Understanding how these reactions occur and, crucially, how their rates can be controlled is of paramount importance in various fields, including chemistry, biology, engineering, and materials science. A key factor determining the speed of a reaction is its activation energy – the energy barrier that must be overcome for reactants to transform into products. This is where catalysts enter the picture. Catalysts significantly accelerate reaction rates by lowering the activation energy, without being consumed in the process itself. But how do they achieve this remarkable feat? This article delves into the intricacies of catalysis, explaining the mechanism by which a catalyst lowers the activation energy and exploring its profound implications.
Understanding Activation Energy
Before diving into the role of catalysts, it's crucial to grasp the concept of activation energy (Ea). Imagine a ball sitting at the top of a hill. To get it to the bottom (representing the products of a reaction), it needs sufficient energy to overcome the hill's height (the activation energy). Similarly, reactant molecules require a minimum amount of energy to initiate a reaction. This energy is used to break existing bonds and initiate the formation of new ones. Molecules possessing this minimum energy are said to be in the activated complex or transition state, a high-energy, unstable intermediate state.
The activation energy determines the reaction rate: a higher activation energy implies a slower reaction rate, as fewer molecules possess the necessary energy to overcome the barrier. Conversely, a lower activation energy translates to a faster reaction rate, as more molecules can readily cross the energy barrier. This dependence is elegantly described by the Arrhenius equation:
k = A * e^(-Ea/RT)
where:
- k is the rate constant
- A is the pre-exponential factor (related to the frequency of collisions)
- Ea is the activation energy
- R is the gas constant
- T is the temperature
This equation clearly shows the inverse exponential relationship between the activation energy and the rate constant – a decrease in Ea leads to a substantial increase in k.
The Catalyst's Role: Providing an Alternative Pathway
Catalysts achieve their rate-enhancing effect by providing an alternative reaction pathway with a lower activation energy. They do not alter the overall thermodynamics of the reaction (ΔG), meaning they don't change the equilibrium position. Instead, they accelerate the approach to equilibrium by facilitating a faster reaction rate. This is achieved through the formation of intermediate complexes between the catalyst and the reactants.
Imagine the hill analogy again. A catalyst is like building a tunnel through the hill, providing a shorter, less steep route for the ball to reach the bottom. This tunnel represents the new, lower-energy pathway facilitated by the catalyst. The reactants interact with the catalyst's surface or active sites, forming an activated complex with a lower energy than the one formed in the uncatalyzed reaction. This lower-energy activated complex subsequently breaks down to form the products, regenerating the catalyst in the process.
Mechanisms of Activation Energy Reduction
The precise mechanism by which a catalyst lowers the activation energy depends on the specific reaction and catalyst involved. However, several general mechanisms are at play:
1. Orientation and Proximity Effects:
Reactant molecules often need to collide with a specific orientation to react effectively. Catalysts can bring reactants into close proximity and orient them favorably, increasing the probability of successful collisions and reducing the energy required for bond breaking and formation. This is particularly important for bimolecular reactions involving two or more reactants.
2. Weak Bonding Interactions:
The catalyst forms weak bonds or interactions with the reactants. These interactions weaken existing bonds within the reactants, making them easier to break and reducing the overall activation energy. The catalyst essentially helps to destabilize the reactants, making them more susceptible to transformation into products.
3. Formation of Intermediate Complexes:
Catalysts often form intermediate complexes with the reactants. These complexes have lower energy than the transition state in the uncatalyzed reaction. The formation and subsequent decomposition of these intermediate complexes constitute the alternative reaction pathway with a lower activation energy. This is a common mechanism in many catalytic processes.
4. Acid-Base Catalysis:
In acid-base catalysis, the catalyst donates or accepts a proton (H+), altering the reactivity of the reactants. This can significantly lower the activation energy by facilitating proton transfer steps that are crucial to the reaction mechanism.
5. Redox Catalysis:
In redox catalysis, the catalyst facilitates electron transfer between reactants, effectively mediating oxidation-reduction reactions. This can lead to a lower activation energy by stabilizing the intermediate oxidation states involved in the reaction.
6. Surface Catalysis:
Heterogeneous catalysts, which are in a different phase from the reactants (e.g., a solid catalyst in a gaseous or liquid reaction), utilize their surface area to provide active sites for reactant adsorption. This increases the concentration of reactants at the catalyst surface, enhancing the probability of successful collisions and lowering the activation energy. The specific arrangement of atoms on the catalyst surface plays a vital role in its catalytic activity.
Examples of Catalytic Reactions
Numerous examples illustrate the role of catalysts in lowering activation energy:
-
Enzymatic reactions: Enzymes, biological catalysts, dramatically accelerate countless biochemical reactions within living organisms. They achieve this by binding to substrates (reactants) and creating an environment favorable for reaction, effectively lowering the activation energy.
-
Haber-Bosch process: This industrial process uses an iron catalyst to synthesize ammonia (NH3) from nitrogen (N2) and hydrogen (H2). The catalyst significantly lowers the activation energy for this crucial reaction, allowing for efficient ammonia production on a large scale.
-
Automotive catalytic converters: These devices utilize transition metal catalysts (platinum, palladium, rhodium) to convert harmful pollutants in exhaust gases (e.g., carbon monoxide, nitrogen oxides) into less harmful substances (e.g., carbon dioxide, nitrogen). The catalysts reduce the activation energy for these oxidation-reduction reactions.
-
Polymerization reactions: Many polymerization reactions require catalysts to initiate and control the growth of polymer chains. These catalysts often lower the activation energy for chain initiation and propagation steps, leading to faster and more efficient polymer synthesis.
Conclusion: The Significance of Lowered Activation Energy
The ability of a catalyst to lower the activation energy is of immense importance in countless chemical processes. By accelerating reaction rates, catalysts enhance efficiency, reduce energy consumption, and enable reactions that would otherwise be impractically slow or impossible under typical conditions. The study of catalysis remains a dynamic and vital area of research, continually yielding new insights into the intricate mechanisms and applications of these remarkable substances. Understanding how catalysts reduce activation energy is crucial for designing new catalysts, optimizing existing processes, and developing sustainable chemical technologies for a wide range of applications. Further research into the specific interactions between catalysts and reactants will continue to refine our understanding and lead to even more innovative advancements in catalysis.
Latest Posts
Latest Posts
-
Why Does Electronegativity Increase From Left To Right
Mar 27, 2025
-
What Is The Lcm Of 25 And 35
Mar 27, 2025
-
What Are All The Factors Of 11
Mar 27, 2025
-
What Is The Factors Of 25
Mar 27, 2025
-
How Much Electrons Does Sodium Have
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about A Catalyst Lowers The Activation Energy Of A Reaction By . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
