Name The 3 Parts Of A Dna Nucleotide
Juapaving
Mar 29, 2025 · 7 min read
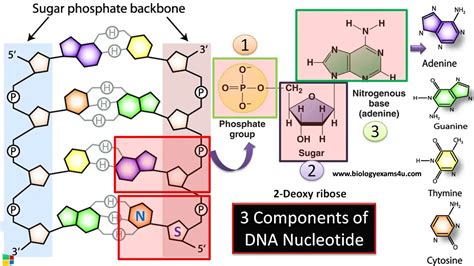
Table of Contents
Name the 3 Parts of a DNA Nucleotide: A Deep Dive into the Building Blocks of Life
Deoxyribonucleic acid, or DNA, is the fundamental blueprint of life. This remarkable molecule holds the genetic instructions for the development, functioning, and reproduction of all known organisms and many viruses. Understanding DNA's structure is crucial to comprehending how life operates at its most basic level. At the heart of this understanding lies the DNA nucleotide – the fundamental building block that makes up this incredible molecule. This article will delve into the three essential components of a DNA nucleotide, exploring their individual characteristics and their collective contribution to the overall structure and function of DNA.
The Triad of a DNA Nucleotide: Phosphate Group, Deoxyribose Sugar, and Nitrogenous Base
Every DNA nucleotide is composed of three distinct parts:
-
A Phosphate Group: This is a negatively charged molecule consisting of a phosphorus atom bonded to four oxygen atoms. It's crucial for the DNA backbone's stability and its interaction with the cellular environment. The negative charges contribute to the overall negative charge of the DNA molecule, influencing its interactions with proteins and other cellular components. The phosphate group acts as a link, connecting nucleotides in a long chain.
-
A Deoxyribose Sugar: This is a five-carbon sugar molecule that forms the structural backbone of the DNA molecule. The "deoxy" prefix signifies the absence of an oxygen atom on the 2' carbon, differentiating it from ribose, the sugar found in RNA. This subtle difference has profound implications for the stability and functionality of DNA compared to RNA. The deoxyribose sugar provides a stable framework for the attachment of the phosphate group and the nitrogenous base. Its specific structure dictates the double-helix shape of DNA.
-
A Nitrogenous Base: This is a nitrogen-containing molecule that comes in four different types in DNA: adenine (A), guanine (G), cytosine (C), and thymine (T). These bases are the informational units of DNA. They pair specifically with each other (A with T, and G with C) through hydrogen bonds, forming the rungs of the DNA ladder. The sequence of these bases along the DNA molecule determines the genetic code, which directs the synthesis of proteins and regulates various cellular processes.
Let's examine each component in detail:
Deep Dive into the Phosphate Group
The phosphate group (PO₄³⁻) is a fundamental component of many biomolecules, not just DNA. Its negative charge is essential for its role in DNA. This charge influences several critical aspects:
-
DNA Stability: The repulsive forces between the negatively charged phosphate groups along the DNA backbone help maintain the double helix structure. This repulsion prevents the molecule from collapsing. Counteracting these forces are positively charged ions (cations) within the cell, which neutralize the negative charges and contribute to DNA stability.
-
Enzyme Interactions: Many enzymes involved in DNA replication, transcription, and repair interact with the phosphate groups. These enzymes recognize and bind to specific regions of DNA based on the structure and charge of the phosphate backbone. For example, DNA polymerases, which synthesize new DNA strands, interact with the phosphate groups to add new nucleotides.
-
DNA Packaging: The negative charge of the phosphate groups plays a role in how DNA is packaged within the cell. The DNA molecule is wound around histone proteins, which are positively charged, neutralizing the negative charges and facilitating compact packaging within the cell nucleus.
Understanding the Deoxyribose Sugar
The deoxyribose sugar is a pentose sugar, meaning it has five carbon atoms. The absence of the hydroxyl (-OH) group on the 2' carbon is a key distinguishing feature between deoxyribose and ribose. This seemingly small difference is crucial for the stability and functionality of DNA:
-
Increased Stability: The absence of the 2'-OH group in deoxyribose makes DNA less susceptible to hydrolysis (breakdown by water). RNA, with its ribose sugar, is much more prone to hydrolysis because of the presence of the 2'-OH group, which makes it less stable than DNA. This increased stability is vital for storing genetic information over long periods.
-
Double Helix Structure: The specific conformation of deoxyribose dictates the geometry of the DNA double helix. The sugar's orientation and bond angles contribute to the overall shape and dimensions of the molecule. This structure is crucial for the specific pairing of nitrogenous bases and the stability of the double helix.
-
Attachment Points: Deoxyribose provides attachment points for both the phosphate group and the nitrogenous base. The 3' and 5' carbon atoms of deoxyribose form the phosphodiester bonds that link adjacent nucleotides in the DNA chain, creating the backbone. The 1' carbon atom of deoxyribose is linked to the nitrogenous base.
The Nitrogenous Bases: The Language of Life
The nitrogenous bases are the information-carrying components of DNA. These are the molecules that actually code for genetic information. Their specific arrangement determines the sequence of amino acids in proteins, and thus, the characteristics of an organism.
-
Purines (Adenine and Guanine): These are larger, double-ring structures. Adenine (A) forms two hydrogen bonds with thymine (T), while guanine (G) forms three hydrogen bonds with cytosine (C).
-
Pyrimidines (Cytosine and Thymine): These are smaller, single-ring structures. Cytosine (C) and thymine (T) pair with guanine (G) and adenine (A), respectively, through hydrogen bonding.
The specific pairing of bases (A with T, and G with C) is dictated by the geometry and the hydrogen-bonding capacity of each base. This complementary base pairing is fundamental to DNA replication and transcription. The sequence of bases along the DNA molecule constitutes the genetic code, which dictates the sequence of amino acids in proteins and the regulation of gene expression. The genetic code is universal, meaning that the same codons (three-base sequences) specify the same amino acids in almost all organisms.
The Phosphodiester Bond: Linking Nucleotides Together
The individual nucleotides are linked together to form a polynucleotide chain through phosphodiester bonds. These bonds form between the 3' hydroxyl group (-OH) of one deoxyribose sugar and the 5' phosphate group of the next deoxyribose sugar. This creates a sugar-phosphate backbone, with the nitrogenous bases projecting inwards. The directionality of the DNA strand is designated as 5' to 3', reflecting the orientation of the phosphodiester bonds.
The phosphodiester bonds are relatively stable, but they can be broken by enzymes during DNA replication and repair. The stability of these bonds is crucial for the long-term stability of the DNA molecule and the integrity of the genetic information it carries.
The Double Helix: Structure and Function
The two polynucleotide chains wind around each other to form the iconic double helix structure. The two strands are antiparallel, meaning that they run in opposite directions (one 5' to 3', and the other 3' to 5'). The nitrogenous bases from the two strands pair through hydrogen bonds, forming the "rungs" of the DNA ladder. The specific pairing of bases (A with T, and G with C) ensures that the two strands are complementary to each other. This complementarity is crucial for DNA replication, where each strand can serve as a template for the synthesis of a new complementary strand.
The double helix structure provides several advantages:
- Protection of Genetic Information: The bases are shielded within the helix, protecting them from chemical damage.
- Compact Packaging: The helical structure allows for efficient packing of the DNA molecule within the cell nucleus.
- Replication and Transcription: The double helix structure facilitates the processes of DNA replication and transcription, which are essential for the transmission of genetic information.
The Importance of Understanding DNA Nucleotides
Understanding the three components of a DNA nucleotide – the phosphate group, the deoxyribose sugar, and the nitrogenous base – is fundamental to grasping the structure and function of DNA. The intricate interplay of these components gives rise to the remarkable properties of DNA: its stability, its ability to store and transmit genetic information, and its role as the fundamental blueprint of life. This understanding is crucial for advancements in fields like genetics, medicine, biotechnology, and many other scientific disciplines. Research continues to uncover more about the intricacies of DNA, and its building blocks will remain central to this ongoing exploration of the secrets of life. The specificity of the interactions between these components is a testament to the elegance and precision of biological systems. Further study into each component can lead to breakthroughs in understanding and treating genetic diseases. Continued research into DNA nucleotide structure and function promises to revolutionize our understanding of life itself.
Latest Posts
Latest Posts
-
A To Z In Cursive Writing
Mar 31, 2025
-
What Is The Purpose Of Mitosis In Single Celled Organisms
Mar 31, 2025
-
What Is The Difference Between A Land And Sea Breeze
Mar 31, 2025
-
A Real Gas Behaves Most Like An Ideal Gas At
Mar 31, 2025
-
How Many Valence Electrons Are Found In Phosphorus
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Name The 3 Parts Of A Dna Nucleotide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
