Mass Of An Electron In Kilograms
Juapaving
Mar 20, 2025 · 6 min read
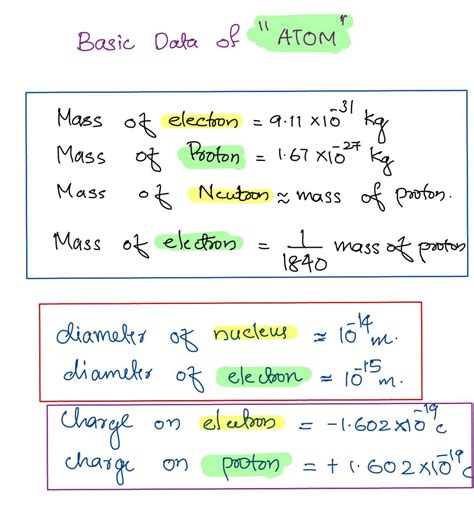
Table of Contents
The Mass of an Electron in Kilograms: A Deep Dive into Subatomic Physics
The electron, a fundamental constituent of matter, holds a pivotal position in the world of physics. Understanding its properties, especially its mass, is crucial to grasping the intricacies of atomic structure, chemical reactions, and the very fabric of the universe. While often expressed in other units, like electron volts (eV) or atomic mass units (amu), the mass of an electron in kilograms provides a relatable, albeit incredibly tiny, measure of its existence. This article delves deep into the electron's mass, exploring its measurement, significance, and implications across various scientific disciplines.
What is the Mass of an Electron in Kilograms?
The accepted value for the rest mass of an electron is approximately 9.10938356 × 10<sup>-31</sup> kilograms. This incredibly small number highlights just how minuscule these fundamental particles are. To put this into perspective, it would take roughly 10<sup>28</sup> electrons to equal the mass of a single grain of sand. The precision of this measurement underscores the remarkable advancements in experimental physics and the sophistication of the techniques used to determine such minute quantities.
Methods for Measuring Electron Mass
Determining the mass of an electron has been a significant challenge throughout the history of physics. Several ingenious methods have been employed, each building upon previous advancements and contributing to the increasingly precise value we have today.
1. Millikan's Oil Drop Experiment
While not directly measuring the electron's mass, Robert Millikan's famous oil drop experiment in 1909 played a crucial role. This experiment determined the elementary charge (e) of an electron with remarkable accuracy. Knowing the charge, scientists could then use other experimental data, such as the charge-to-mass ratio (e/m), to calculate the mass. The charge-to-mass ratio was initially determined through observations of cathode rays.
2. Measuring the Charge-to-Mass Ratio (e/m)
J.J. Thomson's experiments with cathode rays in the late 19th century provided the first determination of the electron's charge-to-mass ratio. By observing the deflection of cathode rays in electric and magnetic fields, he was able to calculate this ratio. This groundbreaking work paved the way for subsequent experiments aimed at determining the mass independently.
3. Advanced Spectroscopic Techniques
Modern techniques leverage advanced spectroscopic methods and high-precision measurements. These methods often involve analyzing the energy levels of atoms and molecules, where the electron's mass plays a crucial role in determining the precise energy transitions. High-resolution spectroscopy allows for extraordinarily precise measurements of these energy levels, leading to increasingly accurate calculations of the electron's mass.
4. Penning Traps
Penning traps are sophisticated devices used to confine charged particles using a combination of electric and magnetic fields. By meticulously measuring the cyclotron frequency of an electron trapped within a Penning trap, scientists can determine its mass with exceptional precision. This technique minimizes external influences and allows for highly accurate measurements.
The Significance of the Electron's Mass
The electron's mass, though seemingly insignificant on a macroscopic scale, has profound implications across various scientific domains:
1. Atomic Structure and Stability
The electron's mass is a fundamental parameter in determining the structure and stability of atoms. The interaction between the electron's mass and the electromagnetic force dictates the energy levels within an atom and influences the chemical properties of elements. The relatively small mass of the electron, compared to the proton and neutron, means that its motion is more easily influenced, leading to interesting quantum mechanical phenomena.
2. Chemical Reactions and Bonding
The mass of the electron plays a critical role in chemical bonding. The distribution of electrons in molecules, governed by their mass and energy levels, determines the strength and nature of chemical bonds. The electron's mass influences the reactivity of elements and compounds, impacting a wide range of chemical processes.
3. Nuclear Physics and Particle Physics
In nuclear physics, the electron's mass is relevant in processes like beta decay, where an electron is emitted from an unstable nucleus. The mass difference between the parent and daughter nuclei provides the energy for this decay. In particle physics, the electron is considered a fundamental lepton, and its mass is crucial in understanding the Standard Model of particle physics and its interactions with other particles.
4. Astrophysics and Cosmology
While seemingly insignificant at the atomic level, the cumulative mass of countless electrons plays a role in the overall mass of stars and galaxies. Understanding the mass and properties of electrons is also crucial in astrophysical phenomena such as stellar nucleosynthesis and the formation of galaxies. The mass influences the radiation emitted and the evolution of celestial bodies.
The Electron's Mass and Relativity
Einstein's theory of special relativity shows that the mass of an object increases with its velocity. While this relativistic mass increase is generally negligible for everyday objects, it becomes significant for particles like electrons that can be accelerated to speeds approaching the speed of light. This relativistic mass increase is incorporated into calculations of electron behavior in high-energy physics experiments. The rest mass we’ve discussed above refers to the mass of the electron at rest.
Units Other Than Kilograms
While kilograms provide a standard unit for mass, the electron's mass is often expressed in other units more suitable for atomic and subatomic scales.
-
Electronvolt (eV): An electronvolt is the energy gained by an electron when it accelerates through a potential difference of one volt. The electron's mass is often expressed as its equivalent energy using Einstein's famous equation, E=mc². This equates to approximately 0.511 MeV (mega-electron volts).
-
Atomic Mass Unit (amu): The atomic mass unit is defined as 1/12 the mass of a carbon-12 atom. The electron's mass is approximately 0.00054858 amu.
Future Research and Refinements
The determination of the electron's mass remains an active area of research. Scientists continue to refine measurement techniques, aiming for even greater accuracy. Improved precision in measuring the electron's mass contributes to a more precise understanding of fundamental physical constants and improves the accuracy of various theoretical models. Future research might involve advanced trapping techniques, new spectroscopic methods, and potentially even entirely novel approaches.
Conclusion
The mass of an electron in kilograms, though an incredibly small number (9.10938356 × 10<sup>-31</sup> kg), is a fundamental constant with far-reaching implications across numerous scientific disciplines. Its accurate measurement has been a significant achievement in physics, relying on innovative experimental techniques and theoretical frameworks. This seemingly minuscule quantity underpins our understanding of atomic structure, chemical bonding, nuclear reactions, and even the vast expanse of the universe. Ongoing research continually refines our understanding of this fundamental particle, contributing to a more complete picture of the physical world. The journey to understand the electron and its mass is a testament to the power of human ingenuity and the relentless pursuit of scientific knowledge.
Latest Posts
Latest Posts
-
What Is The Percentage Of 3 7
Mar 21, 2025
-
Equation Of A Plane Given 3 Points
Mar 21, 2025
-
Why Does Temperature Stay Constant During A Phase Change
Mar 21, 2025
-
What Is The Freezing Point Of Fahrenheit
Mar 21, 2025
-
Is Ba Oh 2 Ionic Or Molecular
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Mass Of An Electron In Kilograms . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
