Is Ba Oh 2 Ionic Or Molecular
Juapaving
Mar 21, 2025 · 5 min read
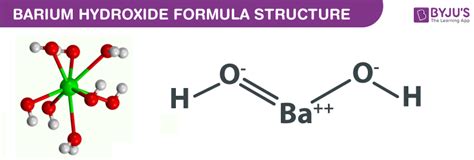
Table of Contents
Is Ba(OH)₂ Ionic or Molecular? A Deep Dive into Chemical Bonding
The question of whether barium hydroxide, Ba(OH)₂, is ionic or molecular is a fundamental one in chemistry, touching upon the core concepts of chemical bonding and the properties of matter. While the answer might seem straightforward at first glance, a deeper understanding requires examining the nature of the constituent ions and the forces holding them together. This article will delve into the intricacies of Ba(OH)₂'s bonding, exploring its structure, properties, and the broader implications for classifying compounds as ionic or molecular.
Understanding Ionic vs. Molecular Compounds
Before we dissect the specifics of Ba(OH)₂, let's clarify the distinction between ionic and molecular compounds. This distinction hinges on the type of bond that holds the constituent atoms or ions together.
Ionic compounds are formed through the electrostatic attraction between positively charged ions (cations) and negatively charged ions (anions). This transfer of electrons results in a strong electrostatic force that holds the ions together in a crystalline lattice structure. These compounds generally exhibit high melting and boiling points, are often soluble in water, and conduct electricity when dissolved or molten.
Molecular compounds, on the other hand, are formed through the sharing of electrons between atoms. This sharing creates covalent bonds, which are generally weaker than ionic bonds. Molecular compounds typically have lower melting and boiling points compared to ionic compounds and often exist as gases, liquids, or low-melting solids. They generally do not conduct electricity in either solid or molten states.
The Case of Barium Hydroxide, Ba(OH)₂
Barium hydroxide, Ba(OH)₂, is a white, crystalline solid. Its chemical formula reveals its constituent parts: barium (Ba), oxygen (O), and hydrogen (H). To determine whether it's ionic or molecular, we must consider the electronegativity differences between the constituent atoms and the nature of the bonding within the compound.
Barium (Ba) is an alkaline earth metal located in Group 2 of the periodic table. Alkaline earth metals readily lose two electrons to achieve a stable noble gas configuration, forming a +2 cation (Ba²⁺). Oxygen (O) and hydrogen (H) together form the hydroxide ion (OH⁻), a polyatomic anion. The hydroxide ion itself exhibits covalent bonding between oxygen and hydrogen, however, the overall compound is primarily considered ionic.
The formation of Ba(OH)₂ involves the transfer of two electrons from the barium atom to the two hydroxide ions. This results in the formation of the Ba²⁺ cation and two OH⁻ anions. The strong electrostatic attraction between these oppositely charged ions forms the ionic bonds that hold the compound together in a crystalline lattice structure.
Therefore, Ba(OH)₂ is primarily considered an ionic compound.
Evidence Supporting the Ionic Nature of Ba(OH)₂
Several properties of Ba(OH)₂ strongly support its classification as an ionic compound:
-
High melting point: Ba(OH)₂ has a relatively high melting point, indicative of strong electrostatic forces between ions requiring significant energy to overcome.
-
Solubility in water: Ba(OH)₂ is soluble in water, a characteristic often associated with ionic compounds. When dissolved in water, the ionic bonds are disrupted, and the Ba²⁺ and OH⁻ ions become hydrated, meaning they are surrounded by water molecules.
-
Conductivity in solution: Aqueous solutions of Ba(OH)₂ conduct electricity. This is because the dissolved Ba²⁺ and OH⁻ ions are free to move and carry an electric charge.
-
Crystalline structure: Ba(OH)₂ exists in a crystalline structure, a characteristic feature of many ionic compounds. This structure is a consequence of the ordered arrangement of ions in a lattice to maximize electrostatic attractions and minimize repulsions.
The Role of the Hydroxide Ion (OH⁻)
The presence of the hydroxide ion (OH⁻) in Ba(OH)₂ warrants further discussion. While the O-H bond within the hydroxide ion is covalent, the overall interaction between Ba²⁺ and OH⁻ is primarily ionic. The covalent bond within the hydroxide ion is significantly stronger than the electrostatic attraction between individual H+ and O2- ions.
The difference in electronegativity between oxygen and hydrogen is substantial enough to form a strong polar covalent bond within the hydroxide ion. However, the entire hydroxide ion acts as a single, negatively charged entity that interacts ionically with the barium cation. This is a crucial distinction. The presence of a covalent bond within a polyatomic ion does not automatically disqualify a compound from being classified as ionic.
Exceptions and Nuances
While Ba(OH)₂ is predominantly ionic, it's important to acknowledge that the concept of ionic vs. molecular is a spectrum rather than a binary classification. Some compounds exhibit characteristics of both ionic and covalent bonding, often referred to as having polar covalent bonds with significant ionic character.
The degree of ionic character in a bond is influenced by the electronegativity difference between the atoms involved. A larger difference leads to a more ionic bond, while a smaller difference suggests a more covalent bond. In Ba(OH)₂, the significant electronegativity difference between Ba and O (and to a lesser extent, H) leads to a predominantly ionic character.
Practical Applications of Ba(OH)₂
Understanding the properties of Ba(OH)₂, stemming from its ionic nature, is crucial for its various applications:
-
Chemical synthesis: Ba(OH)₂ is used as a strong base in various chemical reactions, acting as a reactant or catalyst. Its solubility and reactivity are directly linked to its ionic character.
-
Water treatment: Its alkaline properties make it useful for adjusting the pH of water in various applications.
-
Sugar refining: Ba(OH)₂ is employed in the refining of sugar to remove impurities.
-
Production of other barium compounds: Its ionic nature allows for easy reaction with other compounds to form other barium salts.
Conclusion: Ba(OH)₂ is Primarily Ionic
In conclusion, while the hydroxide ion (OH⁻) within Ba(OH)₂ contains a covalent bond, the overall interaction between the barium cation (Ba²⁺) and the hydroxide anions (OH⁻) is primarily ionic due to the significant electronegativity difference and resulting electron transfer. The high melting point, solubility in water, conductivity in solution, and crystalline structure all strongly support its classification as an ionic compound. The understanding of this distinction is fundamental to comprehending the chemical behavior and applications of Ba(OH)₂. While nuances and exceptions exist within the spectrum of bonding, Ba(OH)₂ firmly resides on the ionic side of the continuum. Understanding the ionic nature of Ba(OH)₂ is crucial for its numerous practical applications across diverse fields.
Latest Posts
Latest Posts
-
Adding And Subtracting Multi Digit Numbers
May 12, 2025
-
In A Nonpolar Covalent Bond Electrons Are
May 12, 2025
-
What Is The Oxidation State Of Sulfur In H2so4
May 12, 2025
-
In Modern Terminology Mendels Heredity Factors Are Called
May 12, 2025
-
How Many Feet In 180 Inches
May 12, 2025
Related Post
Thank you for visiting our website which covers about Is Ba Oh 2 Ionic Or Molecular . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.