What Is The Freezing Point Of Fahrenheit
Juapaving
Mar 21, 2025 · 6 min read
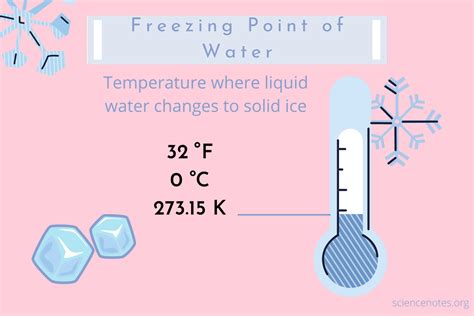
Table of Contents
What is the Freezing Point of Fahrenheit? A Deep Dive into Temperature Scales
The freezing point of water is a fundamental concept in science, crucial for understanding various physical and chemical processes. While many parts of the world utilize the Celsius scale, the Fahrenheit scale remains prevalent, especially in the United States. Understanding the freezing point of water on the Fahrenheit scale is essential for anyone working with temperature measurements or simply seeking a deeper understanding of thermometry. This comprehensive article will delve into the freezing point of Fahrenheit, exploring its history, comparison with other scales, practical applications, and some common misconceptions.
The Freezing Point: 32°F – A Defining Point
The freezing point of water on the Fahrenheit scale is 32°F (degrees Fahrenheit). This is a crucial reference point for the entire scale, serving as the benchmark against which other temperatures are measured. The choice of 32°F for the freezing point wasn't arbitrary; it's rooted in the historical development of the Fahrenheit scale, a story we'll explore in more detail below. Understanding this foundational point allows for accurate conversions to other temperature scales like Celsius and Kelvin, crucial for scientific accuracy and global communication.
The History Behind Fahrenheit's Freezing Point
The Fahrenheit scale, developed by Daniel Gabriel Fahrenheit in the early 18th century, initially used a different reference point for its zero point. Fahrenheit's original scale was based on a mixture of ice, water, and ammonium chloride, which he defined as 0°F. The freezing point of water was subsequently established as 32°F, and the boiling point of water under standard atmospheric pressure as 212°F.
This seemingly arbitrary choice of 0°F and the resulting 32°F freezing point has its roots in practical considerations of the time. The choice of the ice, water, and ammonium chloride mixture provided a readily reproducible and relatively low temperature, offering a convenient starting point for his scale. The range of temperatures he observed within his environment, including body temperature and boiling water, then dictated the other points on the scale. It's vital to understand that the Fahrenheit scale's development was empirical, a testament to the scientific advancements of the era and a foundation upon which more refined scales were built.
Evolution and Standardization
Over time, the Fahrenheit scale underwent refinements, but the freezing point of water remained fixed at 32°F. This standardization has ensured consistency in measurements and comparisons across diverse contexts, from everyday weather reports to scientific experiments. The consistency of this crucial point, despite the initial empirical methods, is a testament to the enduring value of the Fahrenheit scale, even in a world increasingly reliant on the Celsius scale.
Comparing Fahrenheit to Other Scales: Celsius and Kelvin
Understanding the freezing point of Fahrenheit requires comparing it to other widely used temperature scales: Celsius and Kelvin.
Fahrenheit vs. Celsius
The Celsius (or Centigrade) scale uses 0°C for the freezing point of water and 100°C for the boiling point. The conversion between Fahrenheit and Celsius is a well-known formula:
°C = (°F - 32) × 5/9
This formula highlights the relationship between the two scales. The difference of 32°F between the freezing point of water on both scales reflects the historical development of the Fahrenheit scale. Conversely, converting from Celsius to Fahrenheit uses the formula:
°F = (°C × 9/5) + 32
These formulas are essential tools for anyone working with temperature data in both scales, ensuring accurate conversions and comparisons.
Fahrenheit vs. Kelvin
The Kelvin scale, an absolute temperature scale, uses absolute zero (0 K) as its zero point, representing the theoretical absence of all thermal energy. The freezing point of water on the Kelvin scale is 273.15 K. Conversion between Fahrenheit and Kelvin is achieved through a two-step process involving conversion to Celsius first, and then to Kelvin:
- °C = (°F - 32) × 5/9
- K = °C + 273.15
The Kelvin scale is widely used in scientific research, especially in fields like thermodynamics and physics, where absolute temperature is crucial for accurate calculations and modeling. Understanding the relationship between Fahrenheit and Kelvin emphasizes the importance of considering the underlying principles of temperature measurement.
Practical Applications of the Freezing Point in Fahrenheit
The knowledge of the freezing point of water at 32°F finds widespread applications in numerous fields, ranging from everyday life to sophisticated scientific endeavors.
Everyday Applications
-
Food Preservation: Freezing food at or below 32°F is a common method of preserving it, inhibiting bacterial growth and extending its shelf life. Understanding this crucial temperature point is fundamental to safe food handling and preservation.
-
Winter Weather: Weather reports often use Fahrenheit to communicate freezing temperatures, informing individuals about potential hazards like icy roads or frost damage to crops. This information is vital for personal safety and infrastructure maintenance.
-
Plumbing and Construction: The freezing point of water dictates the precautions needed to protect plumbing systems and construction materials during freezing temperatures to avoid damage from expansion of ice.
Scientific and Industrial Applications
-
Material Science: The freezing point of water is critical in many material science experiments and applications. It influences the behavior of materials at different temperatures, including their physical and chemical properties.
-
Chemical Engineering: Chemical reactions often have temperature-dependent reaction rates, with the freezing point of water acting as a key reference point for controlling reaction conditions.
-
Cryogenics: The freezing point of water serves as a comparative point when discussing extremely low temperatures, highlighting the difference between freezing and cryogenic temperatures.
-
Meteorology and Climatology: The freezing point in Fahrenheit is essential for analyzing and modeling weather patterns, helping to predict weather events and understand climate change.
Common Misconceptions about the Freezing Point
While the freezing point of water at 32°F is well-established, some common misconceptions exist:
-
Freezing Point is Always 32°F: The freezing point of water is 32°F only under standard atmospheric pressure. At higher altitudes or pressures, the freezing point can vary slightly.
-
Freezing and Melting are Instantaneous: The transition between liquid water and ice isn't instantaneous. It takes time for the heat transfer required for freezing or melting to occur.
-
All Substances Freeze at 32°F: This is false. Each substance has its unique freezing point, dependent on its physical and chemical properties. Water's freezing point is specific to water.
Conclusion: The Significance of 32°F
The freezing point of water at 32°F is not merely a numerical value; it's a fundamental constant with far-reaching implications across numerous scientific disciplines and everyday applications. Understanding its historical context, its relationship to other temperature scales, and its practical applications provides valuable insight into the world around us. From preventing burst pipes during winter to preserving food safely, the significance of 32°F extends beyond theoretical concepts, impacting our daily lives in profound ways. Continued exploration and understanding of temperature scales, including Fahrenheit, remains essential for technological advancements and ensuring safety and efficiency across various domains. Its lasting presence underscores the importance of adapting and evolving measurement systems to suit both historical context and the need for scientific precision.
Latest Posts
Latest Posts
-
Is Osmosis Low To High Or High To Low
May 12, 2025
-
Show Me A Picture Of A Polygon
May 12, 2025
-
What Does The Slope Of Distance Time Graph Represent
May 12, 2025
-
How To Write A Check For 850
May 12, 2025
-
Do All Birds Have Hollow Bones
May 12, 2025
Related Post
Thank you for visiting our website which covers about What Is The Freezing Point Of Fahrenheit . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.