Litmus Paper Test For Acid And Base
Juapaving
Mar 25, 2025 · 6 min read
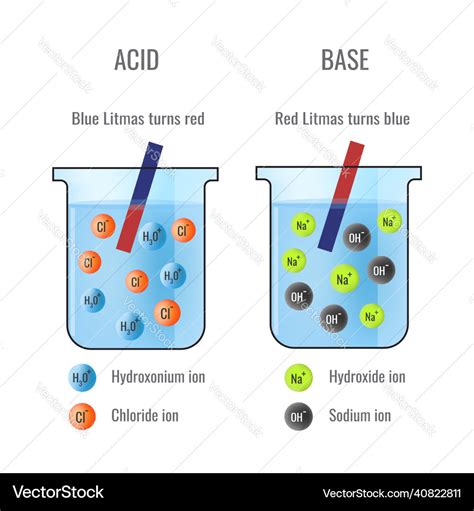
Table of Contents
Litmus Paper Test: A Comprehensive Guide to Identifying Acids and Bases
The litmus paper test is a cornerstone of basic chemistry, providing a simple yet effective method for distinguishing between acidic and alkaline (basic) substances. This versatile test has applications ranging from school science experiments to industrial quality control. Understanding its principles, limitations, and proper execution is crucial for accurate results and safe laboratory practice. This comprehensive guide will delve deep into the litmus paper test, exploring its history, methodology, applications, and the chemistry behind its effectiveness.
Understanding Acids and Bases: A Quick Recap
Before diving into the litmus paper test itself, let's briefly revisit the fundamental concepts of acids and bases. Acids are substances that donate protons (H⁺ ions) in a solution, while bases accept protons. This classic definition, known as the Brønsted-Lowry theory, helps us understand the chemical interactions that underpin the litmus test. Acids generally have a sour taste and can react with metals, producing hydrogen gas. Bases, conversely, often taste bitter and feel slippery.
The pH scale, ranging from 0 to 14, provides a quantitative measure of acidity and basicity. A pH of 7 represents neutrality (pure water). Values below 7 indicate acidity, with lower values representing stronger acids. Values above 7 indicate alkalinity, with higher values representing stronger bases. The litmus paper test gives a qualitative indication of pH, categorizing substances as either acidic or basic.
The History and Chemistry of Litmus Paper
The history of litmus paper traces back centuries. Lichens, a symbiotic organism composed of fungi and algae, have been used for centuries as a source of natural dyes. It was discovered that extracts from certain lichens exhibited a color change depending on the acidity or alkalinity of the solution they were in. This color-changing property forms the basis of the litmus test.
The active components in litmus paper are various organic compounds derived from lichens, collectively referred to as litmus dyes. These dyes are weak acids or bases themselves and undergo structural changes upon reacting with H⁺ or OH⁻ ions in a solution. This structural change alters the way the dye interacts with light, resulting in the observable color change. In acidic solutions, the litmus dye molecules exist in a different form than in alkaline solutions, leading to the distinct color differences.
How to Perform a Litmus Paper Test: A Step-by-Step Guide
Performing a litmus paper test is straightforward:
-
Gather your materials: You'll need litmus paper (both red and blue strips), a sample of the substance you want to test (liquid or solid), a clean glass or plastic container (such as a watch glass or beaker), and distilled water (if necessary).
-
Prepare your sample: If your sample is a solid, you may need to dissolve a small amount in distilled water to create a solution. Ensure the solution is well-mixed.
-
Test with red litmus paper: Dip a strip of red litmus paper into the sample. If the paper turns blue, the substance is basic (alkaline). If no color change occurs, the substance is either neutral or acidic.
-
Test with blue litmus paper: Dip a strip of blue litmus paper into the sample. If the paper turns red, the substance is acidic. If no color change occurs, the substance is either neutral or basic.
-
Interpret your results: The color changes indicate the pH of the substance:
- Red litmus paper turns blue: Basic (alkaline) substance
- Blue litmus paper turns red: Acidic substance
- No color change in either paper: Neutral substance or a very weak acid/base
Important Considerations:
- Small sample size: Use only a small amount of the substance to be tested.
- Cleanliness: Ensure the litmus paper and container are clean to prevent contamination.
- Proper disposal: Dispose of the used litmus paper according to your laboratory’s waste disposal protocol.
Interpreting the Results and Understanding Limitations
While the litmus test provides a quick and easy indication of acidity or alkalinity, it's crucial to understand its limitations:
-
Qualitative, not quantitative: Litmus paper only tells you whether a substance is acidic or basic; it doesn't provide a precise pH value. For quantitative pH measurement, a pH meter is necessary.
-
Sensitivity: The litmus test is not sensitive to very weak acids or bases. A substance might be slightly acidic or basic but not cause a noticeable color change.
-
Interference: Certain substances can interfere with the litmus test, leading to inaccurate results. For example, highly colored solutions might mask the color change of the litmus paper.
Applications of the Litmus Paper Test
The litmus paper test's simplicity and affordability have led to its widespread use across numerous applications:
-
Educational settings: It's a fundamental tool in teaching basic chemistry concepts to students of all ages.
-
Laboratory testing: It's used as a preliminary test in various analytical procedures to quickly screen for acidic or basic substances.
-
Industrial quality control: In numerous industries, litmus paper helps monitor the pH of products and processes, ensuring quality and safety. Examples include the food and beverage industry, water treatment, and chemical manufacturing.
-
Environmental monitoring: It can be used for simple pH tests of soil samples or water bodies, providing a basic assessment of environmental conditions.
-
Home testing kits: Litmus paper is included in many home testing kits for assessing the pH of swimming pools, aquariums, or even soil in gardens.
Safety Precautions When Using Litmus Paper
While litmus paper is generally safe to handle, a few precautions should be observed:
-
Avoid touching the paper with bare hands: Handle the litmus paper by the edges to prevent contamination.
-
Work in a well-ventilated area: Although litmus paper itself is not harmful, the substances being tested might be volatile or corrosive.
-
Wear appropriate personal protective equipment (PPE): Depending on the substance being tested, gloves and eye protection might be necessary.
-
Follow proper disposal procedures: Used litmus paper should be disposed of according to your laboratory or home safety guidelines.
Beyond Litmus: Other Indicators of Acidity and Basicity
While litmus paper is a common indicator, other substances can also be used to detect acids and bases. These include:
-
Phenolphthalein: This indicator is colorless in acidic solutions and turns pink in basic solutions.
-
Methyl orange: This indicator is red in acidic solutions and yellow in basic solutions.
-
Universal indicator: This is a mixture of several indicators that provides a wider range of color changes across the entire pH scale, offering a more precise indication of pH than litmus paper alone.
These indicators, like litmus paper, provide a qualitative measure of pH, but they often have different pH ranges where they show significant color changes. The choice of indicator depends on the specific application and the expected pH range of the substance being tested.
Conclusion: The Enduring Value of the Litmus Test
The litmus paper test, despite its simplicity, remains a valuable tool in chemistry and related fields. Its ease of use, low cost, and widespread availability make it an indispensable method for quickly determining whether a substance is acidic or basic. While its qualitative nature limits its precision compared to more advanced techniques like pH meters, its value as a quick screening method and educational tool remains unquestionable. Understanding its principles, limitations, and proper application is essential for obtaining accurate results and ensuring safe laboratory practices. By combining the knowledge gained from this guide with careful execution, you can effectively utilize litmus paper for diverse applications, from simple classroom experiments to more sophisticated laboratory analyses.
Latest Posts
Latest Posts
-
Cod Chemical Oxygen Demand Vs Bod
Mar 26, 2025
-
How Many Electrons Does Iron Have
Mar 26, 2025
-
Which Molecule Do Plants Use To Store Extra Glucose
Mar 26, 2025
-
What Is The Relationship Between Concentration And Absorbance
Mar 26, 2025
-
Least Common Multiple Of 10 And 20
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Litmus Paper Test For Acid And Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
