How Many Electrons Does Iron Have
Juapaving
Mar 26, 2025 · 6 min read
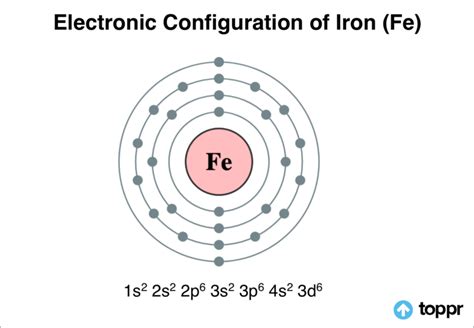
Table of Contents
- How Many Electrons Does Iron Have
- Table of Contents
- How Many Electrons Does Iron Have? Delving into Atomic Structure and Beyond
- Understanding Atomic Structure: Protons, Neutrons, and Electrons
- The Atomic Number of Iron: Unveiling the Electron Count
- Electron Configuration: Orbitals and Shells
- Isotopes of Iron: Variations in Neutrons, Same Electron Count (Usually)
- Ions of Iron: Electron Loss and Gain
- The Biological Significance of Iron and its Electrons
- Industrial Applications of Iron and its Electronic Properties
- Conclusion: The Importance of Understanding Iron's Electron Count
- Latest Posts
- Latest Posts
- Related Post
How Many Electrons Does Iron Have? Delving into Atomic Structure and Beyond
Iron, a ubiquitous element crucial to life and industry, presents a fascinating study in atomic structure. Understanding the number of electrons in an iron atom is fundamental to comprehending its chemical properties, its role in biological systems, and its diverse applications. This article will not only answer the core question – how many electrons does iron have? – but also delve deeper into the intricacies of atomic structure, electron configuration, and the significance of iron's electron arrangement in its various roles.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we pinpoint the electron count for iron, let's establish a basic understanding of atomic structure. An atom is the fundamental building block of matter, consisting of three primary subatomic particles:
- Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element's atomic number and is unique to each element.
- Neutrons: Neutrally charged particles also located in the nucleus. The number of neutrons can vary within an element, resulting in isotopes.
- Electrons: Negatively charged particles that orbit the nucleus in electron shells or energy levels. The number of electrons typically equals the number of protons in a neutral atom.
The Atomic Number of Iron: Unveiling the Electron Count
Iron's atomic number is 26. This crucial number signifies that a neutral iron atom possesses 26 protons in its nucleus. Since the number of electrons in a neutral atom always balances the number of protons to maintain electrical neutrality, a neutral iron atom also possesses 26 electrons.
Therefore, the answer to the core question is: a neutral iron atom has 26 electrons.
Electron Configuration: Orbitals and Shells
While knowing the total number of electrons is important, understanding their arrangement within the atom is crucial for comprehending its chemical behavior. Electrons occupy specific energy levels or shells, and within these shells, they are further organized into orbitals.
The electron configuration of iron is: 1s²2s²2p⁶3s²3p⁶4s²3d⁶.
Let's break this down:
- 1s²: Two electrons occupy the first energy level (shell), specifically the 1s orbital.
- 2s²2p⁶: Eight electrons fill the second energy level, with two in the 2s orbital and six in the 2p orbitals.
- 3s²3p⁶: Another eight electrons reside in the third energy level, distributed similarly to the second level.
- 4s²3d⁶: The remaining six electrons occupy the fourth energy level (4s orbital) and the third energy level (3d orbitals). Note that the 4s orbital fills before the 3d orbitals due to subtle energy level differences.
This specific arrangement of electrons determines iron's reactivity and its ability to form chemical bonds with other elements. The presence of six electrons in the 3d orbital is particularly important, contributing to iron's variable oxidation states and its capacity to participate in a wide range of chemical reactions.
Isotopes of Iron: Variations in Neutrons, Same Electron Count (Usually)
Isotopes are atoms of the same element that have the same number of protons but differ in their number of neutrons. Iron has several naturally occurring isotopes, including ⁵⁴Fe, ⁵⁶Fe, ⁵⁷Fe, and ⁵⁸Fe. The number preceding the element symbol (e.g., ⁵⁶) represents the mass number, the sum of protons and neutrons.
While the number of neutrons varies between iron isotopes, the number of electrons in a neutral iron atom remains consistent at 26 regardless of the isotope. Ions, which are charged atoms, are exceptions and will have a different number of electrons than their proton count.
Ions of Iron: Electron Loss and Gain
Iron readily forms ions, meaning it can lose or gain electrons to achieve a stable electron configuration. The most common oxidation states of iron are +2 (ferrous) and +3 (ferric).
- Fe²⁺ (Ferrous ion): Iron loses two electrons, resulting in a total of 24 electrons.
- Fe³⁺ (Ferric ion): Iron loses three electrons, leaving it with 23 electrons.
These ions play significant roles in various chemical and biological processes.
The Biological Significance of Iron and its Electrons
Iron's 26 electrons are fundamentally linked to its crucial role in biological systems. Iron is an essential element for many living organisms. It is a key component of:
- Hemoglobin: The protein in red blood cells responsible for oxygen transport. The iron atom in the heme group binds to oxygen, enabling its delivery throughout the body. The electron configuration of iron within the heme group facilitates this crucial oxygen-binding process.
- Myoglobin: A protein in muscle tissue that stores oxygen. Similar to hemoglobin, myoglobin utilizes iron's electron properties for oxygen storage and release.
- Cytochromes: Proteins involved in electron transport chains, crucial for cellular respiration and energy production. Iron’s ability to accept and donate electrons is central to their function.
- Enzymes: Numerous enzymes utilize iron as a cofactor, facilitating various metabolic reactions. The electron configuration of iron influences the catalytic activity of these enzymes.
The precise electron configuration of iron within these biological molecules influences their function, efficiency, and overall contribution to biological processes.
Industrial Applications of Iron and its Electronic Properties
Iron's unique electronic structure contributes to its extensive use in industrial applications:
- Steel production: Iron is the primary component of steel, a strong and versatile material used in construction, manufacturing, and countless other applications. The properties of steel are significantly influenced by the addition of other elements that interact with iron's electronic structure.
- Catalysis: Iron and iron-containing compounds are employed as catalysts in various industrial processes, facilitating chemical reactions without being consumed themselves. The electron-donating or electron-accepting capabilities of iron play a key role in catalytic activity.
- Pigments: Iron oxides are widely used as pigments in paints, cosmetics, and other applications. The electronic structure of iron ions influences the color and properties of these pigments.
- Magnetic materials: Iron is a ferromagnetic material, meaning it exhibits strong magnetic properties. This arises from the specific arrangement and interaction of electrons within the iron atoms.
Conclusion: The Importance of Understanding Iron's Electron Count
The seemingly simple question, "How many electrons does iron have?" opens a gateway to a deeper understanding of atomic structure, chemical bonding, and the diverse roles of this essential element. The 26 electrons of a neutral iron atom dictate its chemical reactivity, its participation in biological processes, and its widespread applications in industry. Understanding the electron configuration and the behavior of iron's electrons is fundamental to comprehending the properties and applications of this remarkable element. Further exploration into the intricacies of iron's electron behavior reveals its complex and crucial contributions to the natural world and human endeavors.
Latest Posts
Latest Posts
-
Where In The Cell Does The Krebs Cycle Occur
Mar 30, 2025
-
The Si Unit Of Force Is The
Mar 30, 2025
-
Osmosis Low To High Or High To Low
Mar 30, 2025
-
How To Find The Neutron Number
Mar 30, 2025
-
Germinate Seeds In Dark Or Light
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Does Iron Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
