Cod Chemical Oxygen Demand Vs Bod
Juapaving
Mar 26, 2025 · 7 min read
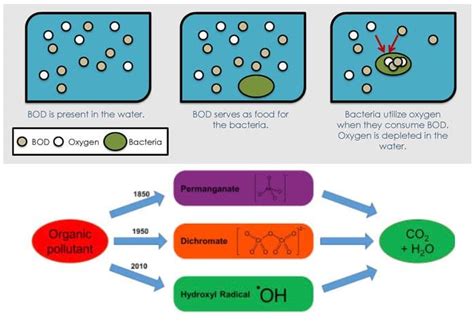
Table of Contents
COD vs BOD: Understanding the Key Differences in Water Quality Analysis
Water quality is paramount for human health and environmental sustainability. Precisely measuring the level of organic pollutants is crucial in safeguarding our water resources. Two essential parameters frequently used for this purpose are Chemical Oxygen Demand (COD) and Biochemical Oxygen Demand (BOD). While both assess the amount of oxygen required to break down organic matter in water, they differ significantly in their methodologies and the information they provide. This article delves into the intricacies of COD and BOD, highlighting their differences, applications, and limitations.
What is Chemical Oxygen Demand (COD)?
Chemical Oxygen Demand (COD) measures the total amount of oxygen required to oxidize all organic and inorganic substances present in a water sample using a strong chemical oxidant. This process typically involves digesting the sample with a strong oxidizing agent, such as potassium dichromate (K₂Cr₂O₇) in a sulfuric acid (H₂SO₄) solution, under reflux conditions at high temperature (usually 150°C). The remaining oxidant is then titrated to determine the amount of oxygen consumed during the oxidation process. This consumed oxygen is directly proportional to the COD value.
Understanding the COD Test Procedure:
The COD test is a relatively straightforward and rapid procedure, typically completed within a few hours. This speed makes it suitable for routine monitoring and immediate assessment of water quality. The process involves:
- Sample Preparation: A precise volume of the water sample is taken and mixed with the oxidizing agent (potassium dichromate) and sulfuric acid.
- Digestion: The mixture is heated to a high temperature (around 150°C) for a specified period, typically 2 hours. This process oxidizes most organic and some inorganic substances. Silver sulfate (Ag₂SO₄) is often added as a catalyst to accelerate the oxidation of certain compounds, particularly volatile fatty acids. Mercury sulfate (HgSO₄) can be added to catalyze the oxidation of chloride ions, minimizing their interference. However, mercury is increasingly avoided due to its environmental toxicity.
- Titration: After cooling, the remaining unreacted dichromate is titrated using a ferrous ammonium sulfate solution. The difference between the initial and final concentrations of dichromate directly indicates the amount of oxygen consumed in the oxidation process.
- Calculation: The COD value is calculated based on the amount of consumed oxygen, usually expressed in milligrams of oxygen per liter of water (mg/L or ppm).
Advantages of COD Testing:
- Speed and Simplicity: COD tests are relatively quick and easy to perform compared to BOD tests.
- Measures Total Oxidizable Material: COD measures virtually all oxidizable organic matter, including both readily biodegradable and non-biodegradable substances.
- Reliable and Reproducible: With proper technique, COD results are highly reliable and reproducible.
Disadvantages of COD Testing:
- Does not Differentiate Biodegradability: COD doesn't distinguish between biodegradable and non-biodegradable organic matter. A high COD value doesn't necessarily indicate a high level of biologically active pollutants.
- High Cost of Reagents: The chemicals used in the COD test can be relatively expensive.
- Potential for Interference: Some inorganic substances can interfere with the COD analysis, requiring pre-treatment or specific modifications of the procedure.
What is Biochemical Oxygen Demand (BOD)?
Biochemical Oxygen Demand (BOD) measures the amount of dissolved oxygen consumed by aerobic microorganisms while they decompose organic matter in a water sample under specific conditions. This process simulates natural degradation in a controlled environment. The BOD test typically involves incubating a diluted water sample in a sealed container at a controlled temperature (usually 20°C) for a specific period (typically 5 days, denoted as BOD₅). The decrease in dissolved oxygen concentration during this period reflects the BOD value.
Understanding the BOD Test Procedure:
The BOD test is a more complex and time-consuming procedure than the COD test. The process typically involves:
- Sample Preparation: A diluted water sample is prepared to ensure sufficient dissolved oxygen for the microorganisms to consume. The dilution factor is critical for accurate results, avoiding oxygen depletion. Seed microorganisms may be added if the sample lacks sufficient microbial diversity for the oxidation of organic substances.
- Incubation: The diluted sample is placed in a sealed bottle and incubated in the dark at a controlled temperature (20°C) for a specified time (usually 5 days).
- Dissolved Oxygen Measurement: The initial and final dissolved oxygen concentrations are measured using an oxygen probe or the Winkler titration method. The difference represents the BOD₅ value.
- Calculation: The BOD value is calculated based on the difference in dissolved oxygen concentration and the dilution factor, also expressed in milligrams of oxygen per liter of water (mg/L or ppm).
Advantages of BOD Testing:
- Reflects Biodegradability: BOD provides a measure of the biodegradable organic matter present in a water sample, reflecting the oxygen demand exerted by biological processes. This directly relates to the impact on aquatic life.
- More Relevant to Aquatic Life: BOD values are more directly relevant to the effects of organic pollution on aquatic ecosystems, as it reflects the actual oxygen consumption by microorganisms.
Disadvantages of BOD Testing:
- Time-Consuming: BOD tests are time-consuming, requiring several days for completion.
- Potential for Errors: Several factors can influence BOD results, including the presence of inhibitory substances, variations in microbial populations, and improper dilution.
- Less Comprehensive: BOD only measures the oxygen demand of readily biodegradable organic substances; it misses refractory compounds that resist microbial degradation.
COD vs. BOD: A Comparative Analysis
| Feature | COD | BOD |
|---|---|---|
| Method | Chemical oxidation | Biological oxidation |
| Time Required | A few hours | 5 days (BOD₅) typically |
| Oxidizing Agent | Potassium dichromate (K₂Cr₂O₇) | Aerobic microorganisms |
| Substances Measured | All oxidizable organic and some inorganic | Primarily biodegradable organic matter |
| Biodegradability | Does not differentiate | Reflects biodegradability |
| Accuracy | High, repeatable | Can be affected by various factors |
| Cost | Relatively high | Relatively low |
| Applications | Routine monitoring, industrial wastewater | Wastewater treatment plant monitoring, environmental assessment |
Applications of COD and BOD
Both COD and BOD are valuable tools in various applications, including:
- Wastewater Treatment: Both tests are crucial for monitoring the effectiveness of wastewater treatment processes. COD provides a quick assessment of the overall organic load, while BOD reflects the oxygen demand imposed by biodegradable pollutants.
- Environmental Monitoring: COD and BOD are used to assess the quality of surface waters, groundwater, and other water bodies. High values indicate potential pollution.
- Industrial Discharge Monitoring: Industries are often required to monitor their wastewater discharges to comply with environmental regulations. COD and BOD are critical parameters in these assessments.
- Research: Both tests are extensively used in research studies related to water quality, pollution control, and environmental remediation.
Choosing Between COD and BOD
The choice between COD and BOD depends on the specific application and the information required. COD is preferred when a rapid assessment of the total organic load is needed, while BOD is more suitable when assessing the biodegradable organic matter and its impact on aquatic life. Often, both tests are used in conjunction to provide a comprehensive understanding of water quality.
Conclusion
COD and BOD are vital parameters for evaluating water quality. While both measure oxygen demand associated with organic matter, they employ different methodologies and provide distinct insights into the nature and extent of organic pollution. Understanding the strengths and limitations of each test is crucial for selecting the appropriate method and interpreting the results accurately. Using both tests in conjunction often provides the most comprehensive evaluation of a water body's health and its capacity to support aquatic life. The development and improvement of these tests are ongoing, aiming for enhanced accuracy, reduced cost, and wider applicability. By carefully considering the specific application and information needs, researchers and practitioners can effectively utilize COD and BOD to safeguard water resources and protect environmental health.
Latest Posts
Latest Posts
-
The Distance Around A Figure Is Called
Mar 29, 2025
-
Is Blood A Compound Or Mixture
Mar 29, 2025
-
What Can 17 Be Divided By
Mar 29, 2025
-
How Many Hearts Does Wormsn Have
Mar 29, 2025
-
Write The Prime Factorization Of 75
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Cod Chemical Oxygen Demand Vs Bod . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
