Is Gold A Pure Substance Or A Mixture
Juapaving
Mar 23, 2025 · 5 min read
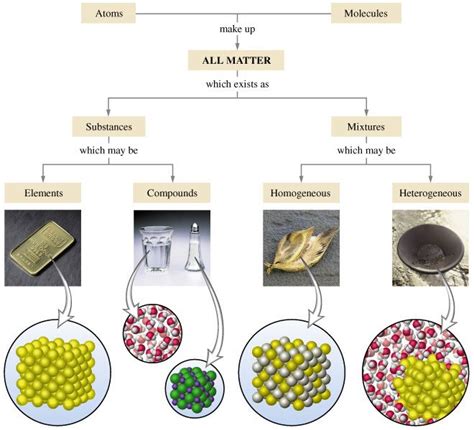
Table of Contents
- Is Gold A Pure Substance Or A Mixture
- Table of Contents
- Is Gold a Pure Substance or a Mixture? Delving into the Nature of Gold
- Understanding Pure Substances and Mixtures
- Pure Substances: The Building Blocks of Matter
- Mixtures: A Blend of Substances
- The Case of Gold: Primarily a Pure Substance
- Gold's Native State: Impurities in Natural Gold
- The Impact of Impurities on Gold's Properties
- Refining Gold: Achieving Higher Purity
- Gold Alloys: Deliberate Mixtures for Enhanced Properties
- Conclusion: A Matter of Perspective
- Latest Posts
- Latest Posts
- Related Post
Is Gold a Pure Substance or a Mixture? Delving into the Nature of Gold
Gold, a highly prized metal throughout history and across cultures, is often perceived as the epitome of purity. However, the scientific classification of gold as a pure substance or a mixture requires a deeper examination of its atomic structure and the potential for impurities. This article will delve into the intricacies of gold's composition, exploring the definitions of pure substances and mixtures, and ultimately answering the question: is gold a pure substance or a mixture?
Understanding Pure Substances and Mixtures
Before we can classify gold, we need to clearly define the terms "pure substance" and "mixture."
Pure Substances: The Building Blocks of Matter
A pure substance is a form of matter that has a constant chemical composition and distinct properties. This means that it is made up of only one type of atom or molecule and cannot be separated into simpler substances by physical means. Pure substances can be either elements or compounds.
-
Elements: These are fundamental substances consisting of only one type of atom. Gold (Au), for instance, is an element, meaning it's composed solely of gold atoms. Other examples include oxygen (O), hydrogen (H), and iron (Fe).
-
Compounds: Compounds are formed when two or more different elements chemically combine in fixed proportions. Water (H₂O), for example, is a compound because it's formed from the chemical bonding of hydrogen and oxygen atoms. Salt (NaCl) is another common example.
Mixtures: A Blend of Substances
A mixture, on the other hand, is a combination of two or more substances that are physically combined but not chemically bonded. The components of a mixture retain their individual properties and can be separated using physical methods like filtration, distillation, or evaporation. Mixtures can be homogeneous or heterogeneous.
-
Homogeneous Mixtures: In a homogeneous mixture, the components are uniformly distributed throughout the mixture. Saltwater is a good example; the salt dissolves completely into the water, resulting in a uniform composition.
-
Heterogeneous Mixtures: Heterogeneous mixtures have components that are not uniformly distributed. A salad, for example, is a heterogeneous mixture because you can clearly distinguish the different ingredients like lettuce, tomatoes, and cucumbers.
The Case of Gold: Primarily a Pure Substance
While the ideal concept of gold is a pure element consisting entirely of gold atoms, naturally occurring gold rarely exists in perfect isolation. The gold we encounter in its natural state often contains trace amounts of other elements, making the question of its purity more nuanced.
Gold's Native State: Impurities in Natural Gold
Gold is often found in its native state, meaning it occurs naturally as a free element, not bound in compounds. However, this naturally occurring gold is rarely 100% pure. It typically contains small quantities of other elements, such as silver, copper, iron, and mercury. These impurities are incorporated into the gold lattice structure during its formation in geological processes. The presence of these trace elements can slightly affect the gold's color, hardness, and other physical properties.
The Impact of Impurities on Gold's Properties
The amount and type of impurities in gold significantly impact its properties. For instance:
-
Color: While pure gold has a characteristic bright yellow color, the presence of other metals can alter its hue. For example, the addition of copper can create a reddish tint, while silver can result in a paler yellow.
-
Hardness: Pure gold is relatively soft and malleable, easily shaped and scratched. Alloying gold with other metals, such as copper or zinc, increases its hardness and durability, making it suitable for jewelry and other applications.
-
Melting Point: The melting point of gold is also slightly affected by the presence of impurities.
Refining Gold: Achieving Higher Purity
The process of refining gold aims to remove these impurities and increase its purity to a very high level. This refining process involves various techniques, including:
-
Cyanide Leaching: This method uses a cyanide solution to dissolve gold from its ore, separating it from other minerals.
-
Electrolytic Refining: This technique involves passing an electric current through a solution containing gold ions, causing pure gold to deposit on a cathode.
-
Miller Process: This method uses chlorine gas to remove impurities from molten gold.
Through refining, gold can achieve purities exceeding 99.99%, often referred to as "four nines" gold. This high purity makes it suitable for various high-tech applications requiring exceptional electrical conductivity and chemical inertness.
Gold Alloys: Deliberate Mixtures for Enhanced Properties
The concept of a gold "alloy" represents a deliberate mixture, where gold is intentionally combined with other metals to improve its properties. These alloys are used extensively in jewelry, electronics, and dentistry. The most common gold alloys include:
-
Gold-Silver Alloys: These alloys offer a lighter color than pure gold and enhance its malleability.
-
Gold-Copper Alloys: Adding copper increases the hardness and durability of gold, making it suitable for jewelry and coins.
-
Gold-Palladium Alloys: These alloys are favored in electronics for their high conductivity and resistance to corrosion.
-
Gold-Nickel Alloys: These alloys have a high tensile strength and are utilized for specific industrial applications.
These gold alloys are mixtures by definition, as they consist of a combination of gold and other elements, held together by metallic bonding. However, it's important to note that the gold itself within the alloy could be considered relatively pure, especially in high-karat alloys.
Conclusion: A Matter of Perspective
The question of whether gold is a pure substance or a mixture is a matter of perspective and the level of detail considered.
At the atomic level, pure gold is a pure substance, composed solely of gold atoms (Au). However, naturally occurring gold invariably contains trace amounts of other elements as impurities. In its natural state, therefore, it is more accurately described as a mixture, albeit one with a predominantly gold composition. The degree of purity can vary significantly depending on its source and refining processes.
Gold alloys, intentionally created mixtures of gold and other metals, are definitively mixtures, designed to modify the characteristics of gold for specific applications. When dealing with refined gold at a very high purity, the term pure substance is appropriate. However, when considering the naturally occurring form or alloys, the term mixture provides a more accurate description. The precise classification ultimately depends on the context and the level of impurity tolerance considered.
Latest Posts
Latest Posts
-
What Are The Factors Of 89
Mar 26, 2025
-
Line Of Best Fit Equation Calculator
Mar 26, 2025
-
What Is The Half Of 24
Mar 26, 2025
-
Is The Number 23 Prime Or Composite
Mar 26, 2025
-
Is Square Root Of 15 A Rational Number
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Is Gold A Pure Substance Or A Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
