Is Calcium A Metal Nonmetal Or Metalloid
Juapaving
Mar 31, 2025 · 6 min read
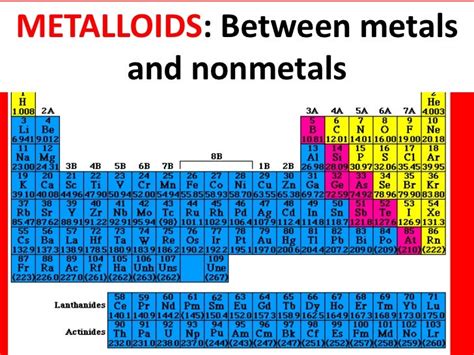
Table of Contents
Is Calcium a Metal, Nonmetal, or Metalloid? A Deep Dive into Calcium's Properties
Calcium, a vital element for life as we know it, sits comfortably within the periodic table's alkaline earth metal family. But what exactly makes it a metal? This article delves deep into the properties of calcium, definitively establishing its metallic nature and exploring its characteristics within the broader context of the periodic table's organization. We'll examine its physical and chemical properties, comparing them to nonmetals and metalloids to solidify its classification as a metal.
Understanding the Periodic Table's Organization
Before we dive into the specifics of calcium, let's refresh our understanding of the periodic table's organization. The table arranges elements based on their atomic number (number of protons) and recurring chemical properties. This arrangement reveals trends in properties, allowing us to predict the behavior of elements based on their position. The table is broadly divided into metals, nonmetals, and metalloids, each exhibiting distinct characteristics.
Metals, located primarily on the left and center of the periodic table, are generally characterized by:
- Good conductors of heat and electricity: Their electrons are loosely held, enabling easy movement and transfer of energy.
- Malleability and ductility: They can be hammered into sheets (malleability) and drawn into wires (ductility) without breaking.
- Lustrous appearance: Many metals possess a shiny, reflective surface.
- High density: They generally have high mass per unit volume.
- Tendency to lose electrons (positive ions): This contributes to their reactivity and ability to form ionic bonds.
Nonmetals, found on the upper right of the periodic table, contrast sharply with metals:
- Poor conductors of heat and electricity: Their electrons are tightly bound, hindering their movement.
- Brittle solids: They tend to shatter easily when subjected to stress.
- Dull appearance: They lack the lustrous sheen of metals.
- Low density: They generally have low mass per unit volume.
- Tendency to gain electrons (negative ions): They readily accept electrons to achieve a stable electron configuration.
Metalloids, also known as semimetals, occupy a transitional zone between metals and nonmetals. They exhibit properties of both groups:
- Intermediate conductivity: Their conductivity is between that of metals and nonmetals, often varying with temperature and other factors.
- Semiconductor properties: Many metalloids are semiconductors, meaning their conductivity can be controlled.
- Brittle solids: Similar to nonmetals, they are often brittle.
- Variable appearance: Their appearance can vary, exhibiting characteristics of both metals and nonmetals.
Calcium's Defining Metallic Properties
Now, let's examine calcium's properties to see where it fits:
1. Electrical Conductivity: Calcium is a good conductor of electricity. Its loosely held valence electrons allow for easy electron flow, a defining characteristic of metals. This conductivity is essential in various applications, including batteries and electrical wiring (although calcium is not commonly used in these roles due to its reactivity with air and moisture).
2. Thermal Conductivity: Similarly, calcium exhibits high thermal conductivity. It efficiently transfers heat, consistent with the metallic nature of its electron structure. This property is less exploited commercially but reflects its metallic character.
3. Malleability and Ductility: While not as malleable or ductile as some other metals like gold or copper, calcium displays these properties to a significant degree. It can be hammered into sheets and drawn into wires, albeit with limitations due to its reactivity and relatively low strength.
4. Metallic Luster: Calcium, in its pure form, exhibits a silvery-white, lustrous appearance. This shine is characteristic of metals and arises from the interaction of light with its delocalized electrons. However, this luster is easily lost due to oxidation in the presence of air.
5. Density: Calcium possesses a relatively low density compared to some other metals, but it's still significantly higher than typical nonmetals. This density is consistent with its atomic structure and metallic bonding.
6. Ionization Energy: Calcium has a relatively low ionization energy. This means that it readily loses electrons to form a stable +2 ion (Ca²⁺). This tendency to lose electrons is a hallmark of metals, enabling them to form ionic compounds with nonmetals.
7. Reactivity: Calcium is a reactive metal, readily reacting with water and acids to produce hydrogen gas and the corresponding calcium salt. This reactivity is again a consequence of its low ionization energy and willingness to lose electrons to achieve a stable electron configuration.
8. Bonding: Calcium primarily forms metallic bonds. Its valence electrons are delocalized, creating a "sea" of electrons that hold the positively charged calcium ions together. This type of bonding is unique to metals and accounts for many of their characteristic properties.
Distinguishing Calcium from Metalloids and Nonmetals
Let's explicitly compare calcium's properties to those of metalloids and nonmetals to further cement its metallic classification:
Calcium vs. Metalloids: Metalloids like silicon and germanium exhibit intermediate electrical conductivity, which is dependent on temperature and other factors. Calcium, however, shows consistent high conductivity, a key differentiator. Moreover, calcium's malleability and ductility are far more pronounced than in brittle metalloids.
Calcium vs. Nonmetals: The differences between calcium and nonmetals are even more striking. Nonmetals are generally poor conductors of heat and electricity, brittle, and have a dull appearance. Calcium's high conductivity, malleability, luster, and reactive nature directly contradict these nonmetal characteristics.
Calcium's Importance and Applications
Understanding calcium's metallic properties is crucial because they dictate its applications and biological roles. While not commonly used in electrical wiring due to its reactivity, calcium's metallic properties play a role in its use in:
- Alloys: Calcium is added to some alloys to improve their properties, such as their workability or strength.
- Reducing agent: Its reactivity makes it a useful reducing agent in the extraction of some metals.
- Deoxidizer: Calcium can remove oxygen from molten metals during processing.
- Biological roles: Calcium ions (Ca²⁺) are vital in many biological processes, such as muscle contraction, nerve impulse transmission, and bone formation. Its metallic properties enable its ability to readily form ionic bonds and participate in these reactions.
Conclusion: Calcium's Unmistakable Metallic Identity
Based on the comprehensive examination of its physical and chemical properties, including its electrical and thermal conductivity, malleability, luster, reactivity, and bonding characteristics, it is undeniable that calcium is a metal. Its properties clearly align with the defining characteristics of metals, setting it apart from both nonmetals and metalloids. Understanding calcium's metallic nature is essential not only for appreciating its place in the periodic table but also for comprehending its vital roles in various industrial and biological processes. Its reactivity, while posing challenges in certain applications, also underscores its intrinsic metallic properties and their influence on its behavior. The unique combination of these properties makes calcium a crucial element for life and various industrial processes.
Latest Posts
Latest Posts
-
What Are The Common Multiples Of 3 And 5
Apr 01, 2025
-
Lcm Of 2 And 3 And 6
Apr 01, 2025
-
What Is The Prime Factorization 81
Apr 01, 2025
-
No Name This Compound According To Iupac Nomenclature Rules Responses
Apr 01, 2025
-
Why Does Heat Not Transfer Through Solids By Convection
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Is Calcium A Metal Nonmetal Or Metalloid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
