Is Boiling An Endothermic Or Exothermic Process
Juapaving
Mar 24, 2025 · 5 min read
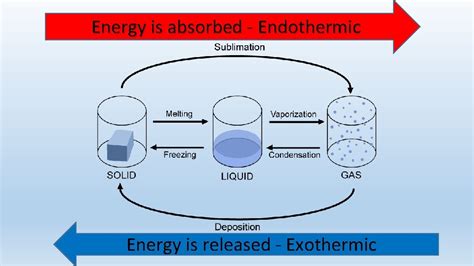
Table of Contents
Is Boiling an Endothermic or Exothermic Process? A Deep Dive
Understanding whether boiling is an endothermic or exothermic process is crucial for grasping fundamental concepts in thermodynamics. While the answer might seem straightforward, a thorough examination reveals a more nuanced understanding of the energy changes involved. This article will delve into the intricacies of boiling, exploring the energy transfers and clarifying the correct classification of this common phase transition.
Understanding Endothermic and Exothermic Processes
Before we delve into the specifics of boiling, let's establish a clear understanding of the terms "endothermic" and "exothermic."
Endothermic processes absorb energy from their surroundings. This absorption of energy manifests as a decrease in the temperature of the surroundings. Think of it as the system "taking in" heat. The enthalpy change (ΔH) for an endothermic process is positive.
Exothermic processes release energy into their surroundings. This release of energy increases the temperature of the surroundings. The system "gives off" heat. The enthalpy change (ΔH) for an exothermic process is negative.
Examples of endothermic processes include melting ice, evaporating water, and photosynthesis. Examples of exothermic processes include combustion, neutralization reactions, and the formation of many ionic compounds.
The Boiling Process: A Closer Look
Boiling is the rapid vaporization of a liquid, occurring when the liquid's vapor pressure equals the surrounding atmospheric pressure. This transition from liquid to gas requires a significant input of energy. The energy is used to overcome the intermolecular forces holding the liquid molecules together, allowing them to escape into the gaseous phase.
Key Factors Influencing Boiling:
- Temperature: The temperature of the liquid must reach its boiling point before boiling can occur. The boiling point is the temperature at which the vapor pressure of the liquid equals the external pressure.
- Pressure: Lower atmospheric pressure results in a lower boiling point. This is why water boils at a lower temperature at high altitudes where the atmospheric pressure is lower.
- Intermolecular Forces: The strength of intermolecular forces (such as hydrogen bonds, dipole-dipole interactions, and London dispersion forces) significantly impacts the boiling point. Stronger intermolecular forces require more energy to overcome, resulting in a higher boiling point.
- Impurities: The presence of impurities in a liquid can slightly affect its boiling point. Generally, impurities elevate the boiling point (boiling point elevation).
The Energy Transfer in Boiling
To understand the energy transfer during boiling, let's consider the molecules within a liquid. These molecules are constantly moving and colliding with each other. At lower temperatures, the kinetic energy of these molecules is insufficient to overcome the intermolecular forces holding them together in the liquid phase.
As we heat the liquid, we increase the average kinetic energy of the molecules. When the kinetic energy of some molecules exceeds the intermolecular forces, they escape from the liquid's surface and enter the gaseous phase. This is evaporation.
Boiling vs. Evaporation: Boiling and evaporation are both phase transitions from liquid to gas. However, they differ in the location where the transition occurs. Evaporation happens only at the surface of the liquid, while boiling occurs throughout the entire liquid volume. Both processes, however, require an input of energy.
Is Boiling Endothermic or Exothermic? The Definitive Answer
Given the explanation above, the answer is clear: boiling is an endothermic process. Energy must be supplied to the liquid to overcome the intermolecular forces and transition the molecules to the gaseous phase. This energy input results in a positive enthalpy change (ΔH > 0). The surrounding environment loses energy to the boiling liquid, resulting in a decrease in the temperature of the environment unless heat is continuously supplied.
Illustrative Examples and Applications
The endothermic nature of boiling manifests itself in numerous everyday occurrences and industrial applications:
- Cooking: Boiling water for cooking pasta or vegetables requires a continuous supply of heat energy. The heat energy is absorbed by the water, converting it from liquid to vapor.
- Steam Generation: Power plants utilize the endothermic nature of boiling to generate steam, which then drives turbines to produce electricity. Large amounts of heat are needed to boil vast quantities of water.
- Refrigeration: Refrigerants utilize the endothermic nature of boiling to absorb heat from the surrounding environment, thereby cooling the space. The refrigerant boils at a low temperature, absorbing heat and creating a cooling effect.
- Distillation: Distillation relies on the different boiling points of liquids to separate mixtures. The process requires heating the mixture, causing the component with the lower boiling point to vaporize and then condense separately.
Further Considerations: Heat of Vaporization
The amount of energy required to vaporize one mole of a liquid at its boiling point is known as the heat of vaporization (ΔH<sub>vap</sub>). This value is a characteristic property of each substance and reflects the strength of its intermolecular forces. Substances with stronger intermolecular forces have higher heats of vaporization. Water, for example, has a relatively high heat of vaporization because of its strong hydrogen bonds. This high heat of vaporization contributes to its effectiveness as a coolant.
Misconceptions and Common Errors
A common misconception is that boiling is exothermic because it produces steam, which can feel hot. The heat felt from steam is not because the boiling process itself is exothermic. Instead, it's due to the steam condensing on cooler surfaces, releasing its latent heat of vaporization. This condensation is an exothermic process. The boiling process itself remains endothermic.
Conclusion: A Comprehensive Understanding
Boiling, unequivocally, is an endothermic process. Understanding this fundamental principle is critical in many areas, from cooking to industrial processes. The energy input required to overcome intermolecular forces and convert a liquid to a gas underscores the endothermic nature of the phase transition. While the heat released during condensation of the steam might seem contradictory, the boiling process itself remains a clear example of an endothermic reaction, constantly demanding a supply of energy to sustain the phase transition. This detailed explanation should help clarify any lingering confusion and enhance your understanding of thermodynamics and phase transitions. Remember to always consider the specific process—boiling itself versus the condensation of the resulting vapor—when analyzing energy changes.
Latest Posts
Latest Posts
-
What Is 4 9 As A Percent
Mar 26, 2025
-
Lowest Common Multiple Of 28 And 42
Mar 26, 2025
-
Moment Of Inertia Of A Wheel
Mar 26, 2025
-
What Is 120 Cm In Inches
Mar 26, 2025
-
At What Temperature Does Your Blood Boil
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Is Boiling An Endothermic Or Exothermic Process . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
