Is Air A Compound Or A Mixture
Juapaving
Mar 25, 2025 · 5 min read
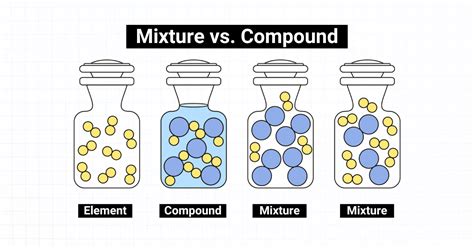
Table of Contents
Is Air a Compound or a Mixture? Understanding the Composition of Our Atmosphere
The question of whether air is a compound or a mixture is a fundamental one in chemistry, with implications for understanding the Earth's atmosphere and its role in supporting life. While the answer might seem simple at first glance, a deeper exploration reveals a nuanced understanding of the nature of air and the distinctions between compounds and mixtures. This comprehensive guide will delve into the chemical composition of air, exploring the arguments for classifying it as a mixture and clarifying the key differences between compounds and mixtures.
The Composition of Air: A Complex Mixture
Air, the gaseous mixture that surrounds our planet, is far from homogeneous. It's a dynamic blend of various gases, with their proportions varying slightly depending on location, altitude, and other factors. The major components of dry air, by volume, are:
- Nitrogen (N₂): Approximately 78%. This diatomic molecule is relatively inert and plays a crucial role in maintaining the balance of the Earth's atmosphere.
- Oxygen (O₂): Approximately 21%. Essential for respiration in most living organisms, oxygen is a highly reactive diatomic molecule.
- Argon (Ar): Approximately 0.93%. This inert noble gas is a significant component, although largely unreactive.
- Carbon Dioxide (CO₂): Approximately 0.04%. While a relatively small component, carbon dioxide plays a vital role in the Earth's climate system through the greenhouse effect.
- Trace Gases: These include neon, helium, methane, krypton, hydrogen, nitrous oxide, and xenon, present in much smaller quantities.
In addition to these gases, air also contains variable amounts of water vapor (H₂O), which can significantly influence atmospheric pressure and humidity. The presence of dust particles, pollen, and other aerosols further adds to the complexity of air's composition.
The Defining Difference: Compounds vs. Mixtures
To definitively answer whether air is a compound or a mixture, we must first understand the key distinctions between these two fundamental concepts in chemistry:
Compounds:
- Fixed Composition: Compounds are formed when two or more elements chemically combine in a fixed, definite proportion. This fixed ratio is expressed by the chemical formula (e.g., H₂O for water).
- Chemical Bonds: The constituent elements are held together by strong chemical bonds (ionic or covalent). These bonds require significant energy to break.
- New Properties: Compounds exhibit properties that are distinctly different from the properties of their constituent elements. For example, water (H₂O) is a liquid at room temperature, whereas hydrogen and oxygen are both gases.
Mixtures:
- Variable Composition: Mixtures consist of two or more substances physically combined, with no fixed ratio of components. The composition can vary greatly depending on the preparation method.
- No Chemical Bonds: The components of a mixture are not held together by chemical bonds. They retain their individual properties.
- Retention of Properties: The properties of a mixture are typically a blend of the properties of its components. For example, saltwater retains the salty taste of sodium chloride and the properties of water.
Why Air is Classified as a Mixture
Based on the defining characteristics of compounds and mixtures, the evidence overwhelmingly supports classifying air as a mixture. Here's why:
- Variable Composition: As previously discussed, the proportions of gases in air vary depending on location and other factors. This variable composition is a hallmark of mixtures.
- No Fixed Ratio: There is no fixed ratio of nitrogen, oxygen, and other gases in air. The relative abundance of each gas is not determined by any chemical formula.
- Retention of Properties: The individual gases in air retain their characteristic properties. For example, oxygen supports combustion, nitrogen is inert, and argon is also inert. These individual properties are not masked or altered in the mixture.
- Physical Separation: The components of air can be physically separated through various methods such as fractional distillation (used to separate the components of liquid air), membrane separation techniques, and condensation. This physical separation is another strong indication that air is a mixture.
The Importance of Air's Composition and its Impact on Life
The specific composition of air is crucial for supporting life on Earth. Oxygen's role in respiration is paramount, providing the necessary oxidant for energy production in most organisms. Nitrogen, while relatively inert, is an essential nutrient for plant growth, converted into usable forms by nitrogen-fixing bacteria. Carbon dioxide plays a crucial role in photosynthesis, the process by which plants convert sunlight into energy.
However, human activities are significantly altering the composition of air, primarily through the release of greenhouse gases like carbon dioxide, methane, and nitrous oxide. This increase in greenhouse gases is driving climate change, with profound implications for ecosystems and human societies. Understanding the composition of air and its changes is critical for addressing environmental challenges and promoting sustainability.
Air as a Homogeneous Mixture
While air is a mixture, it's crucial to note that it's generally considered a homogeneous mixture. This means that its composition is relatively uniform throughout, at least on a macroscopic scale. You wouldn't typically notice distinct layers or regions of different gas concentrations within a sample of air. This homogeneity contrasts with heterogeneous mixtures, such as sand and water, where different components are easily distinguishable.
Conclusion: Air – A Dynamic and Vital Mixture
In conclusion, air is unequivocally classified as a mixture, not a compound. Its variable composition, lack of fixed ratios, retention of individual component properties, and the ease of physical separation of its components all solidify this classification. Understanding air's composition as a dynamic and complex mixture is crucial for comprehending its role in sustaining life on Earth and for addressing environmental challenges related to its changing composition. The ongoing study of air's composition and its interactions with the environment remains a vital area of research in atmospheric science and environmental chemistry. The future of our planet hinges on our ability to understand and manage the intricate interplay of the gases that make up our atmosphere. Continuous monitoring and responsible stewardship of our air are essential for preserving the delicate balance that sustains life on Earth.
Latest Posts
Latest Posts
-
What Is The Smallest Multiple Of 3 And 4
Mar 26, 2025
-
Steroid Hormones Exert Their Action By
Mar 26, 2025
-
Electronic Configuration Of First 20 Elements
Mar 26, 2025
-
All Of The Properties In Math
Mar 26, 2025
-
91 Inches Is How Many Feet
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Is Air A Compound Or A Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
