Electronic Configuration Of First 20 Elements
Juapaving
Mar 26, 2025 · 5 min read
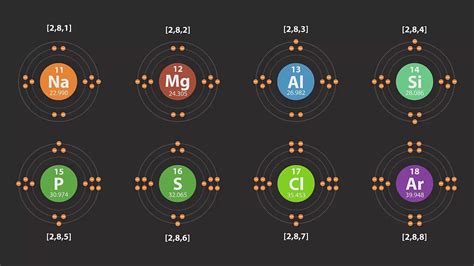
Table of Contents
Electronic Configuration of the First 20 Elements: A Deep Dive
Understanding electronic configuration is fundamental to grasping the behavior of elements and their position within the periodic table. This comprehensive guide delves into the electronic configurations of the first twenty elements, explaining the underlying principles, exceptions, and their implications. We'll explore the Aufbau principle, Hund's rule, and the Pauli exclusion principle – the guiding forces behind electron arrangement.
Understanding Electronic Configuration
Electronic configuration describes how electrons are distributed within the various energy levels and sublevels of an atom. This arrangement dictates an element's chemical properties, reactivity, and its place in the periodic table. It's crucial to understand that electrons don't simply occupy any available space; they follow specific rules governed by quantum mechanics.
Key Principles Governing Electronic Configuration
-
Aufbau Principle: Electrons fill atomic orbitals in order of increasing energy levels. This means lower energy levels are filled first before electrons move to higher energy levels. The order of filling is generally: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, and so on. However, there are exceptions to this rule, which we'll examine later.
-
Pauli Exclusion Principle: A maximum of two electrons can occupy a single atomic orbital, and these two electrons must have opposite spins (one spin-up, one spin-down). This principle is crucial in determining the maximum number of electrons each subshell can hold.
-
Hund's Rule: When filling orbitals within a subshell (like the p or d subshells), electrons will individually occupy each orbital within that subshell before pairing up in any one orbital. This minimizes electron-electron repulsion and leads to greater stability.
Electronic Configurations of the First 20 Elements
Let's explore the electronic configurations of the first twenty elements, highlighting any exceptions and explaining the reasoning behind them:
1. Hydrogen (H): 1s¹ Hydrogen has only one electron, which occupies the lowest energy level, the 1s orbital.
2. Helium (He): 1s² Helium has two electrons, both filling the 1s orbital with opposite spins, completing this energy level. Helium is a noble gas due to its full electron shell.
3. Lithium (Li): 1s²2s¹ With three electrons, lithium begins filling the next energy level, the 2s orbital.
4. Beryllium (Be): 1s²2s² Beryllium fills the 2s orbital, again demonstrating the Pauli Exclusion Principle.
5. Boron (B): 1s²2s²2p¹ Boron begins filling the 2p subshell. Remember, the p subshell can hold a maximum of six electrons (three orbitals, each holding two electrons).
6. Carbon (C): 1s²2s²2p² Carbon has two electrons in the 2p subshell, each occupying a separate orbital according to Hund's rule.
7. Nitrogen (N): 1s²2s²2p³ Nitrogen has three electrons in the 2p subshell, each in a separate orbital, illustrating Hund's rule perfectly.
8. Oxygen (O): 1s²2s²2p⁴ Oxygen begins pairing electrons in the 2p orbitals, as dictated by Hund's rule.
9. Fluorine (F): 1s²2s²2p⁵ Fluorine has only one unpaired electron in the 2p subshell.
10. Neon (Ne): 1s²2s²2p⁶ Neon completes the 2p subshell, resulting in a stable, noble gas configuration.
11. Sodium (Na): 1s²2s²2p⁶3s¹ Sodium begins filling the 3s orbital, starting a new energy level. Notice the stable inner shell configuration mirroring neon.
12. Magnesium (Mg): 1s²2s²2p⁶3s² Magnesium completes the 3s orbital.
13. Aluminum (Al): 1s²2s²2p⁶3s²3p¹ Aluminum initiates filling the 3p subshell.
14. Silicon (Si): 1s²2s²2p⁶3s²3p² Silicon continues filling the 3p subshell.
15. Phosphorus (P): 1s²2s²2p⁶3s²3p³ Phosphorus has three unpaired electrons in the 3p subshell, illustrating Hund's rule.
16. Sulfur (S): 1s²2s²2p⁶3s²3p⁴ Sulfur starts pairing electrons in the 3p orbitals.
17. Chlorine (Cl): 1s²2s²2p⁶3s²3p⁵ Chlorine has one unpaired electron in the 3p subshell.
18. Argon (Ar): 1s²2s²2p⁶3s²3p⁶ Argon completes the 3p subshell, resulting in a stable, noble gas configuration.
19. Potassium (K): 1s²2s²2p⁶3s²3p⁶4s¹ Potassium surprisingly starts filling the 4s orbital before the 3d orbital. This is an exception to the simple Aufbau principle, due to subtle differences in energy levels. The 4s orbital has a slightly lower energy than the 3d orbital in this case.
20. Calcium (Ca): 1s²2s²2p⁶3s²3p⁶4s² Calcium completes the 4s orbital.
Exceptions and Orbital Energy Levels
The seemingly straightforward Aufbau principle has exceptions, particularly with elements involving d and f orbitals. The relative energies of orbitals can change depending on the number of protons and electrons present. The 4s orbital, for example, is lower in energy than the 3d orbital for potassium and calcium. This is due to the shielding effect of inner electrons and the penetration of the 4s orbital closer to the nucleus. This is why the 4s orbital is filled before the 3d orbital. Similar exceptions exist further down the periodic table involving f orbitals.
Significance of Electronic Configuration
The electronic configuration is not just an abstract concept; it's a powerful tool for understanding several key aspects of an element:
-
Chemical Reactivity: Elements with incomplete outer electron shells (valence electrons) are generally more reactive than those with complete shells (noble gases). The number and arrangement of valence electrons directly determine how an element will bond with other atoms.
-
Periodicity: The periodic table itself is organized based on electronic configuration. Elements in the same group (column) have similar outer electron configurations, leading to similar chemical properties.
-
Spectroscopy: Electronic transitions between different energy levels are responsible for the absorption and emission of light by atoms. This principle is fundamental to spectroscopic techniques used in various fields, including astronomy and analytical chemistry.
-
Bonding: Understanding electronic configuration is crucial for predicting the types of bonds (ionic, covalent, metallic) that an element will form.
Conclusion
The electronic configuration of the first twenty elements provides a solid foundation for understanding the principles that govern electron arrangement in atoms. While the Aufbau principle provides a general guideline, remembering the exceptions and underlying energy level interactions is crucial for a complete comprehension. Mastering this fundamental concept unlocks a deeper understanding of the periodic table, chemical bonding, and the fascinating world of atomic structure. By understanding the electronic configurations of elements, we can predict their reactivity, bonding behavior, and spectral properties, making it a cornerstone of chemistry and related fields.
Latest Posts
Latest Posts
-
How Much Valence Electrons Does Oxygen Have
Mar 29, 2025
-
What Percent Of 80 Is 12
Mar 29, 2025
-
Least Common Multiple Of 6 7 And 8
Mar 29, 2025
-
What Is 7 8 Expressed As A Percent
Mar 29, 2025
-
How To Write 200 In Words
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Electronic Configuration Of First 20 Elements . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
