In An Endothermic Reaction Energy Is
Juapaving
Mar 30, 2025 · 5 min read
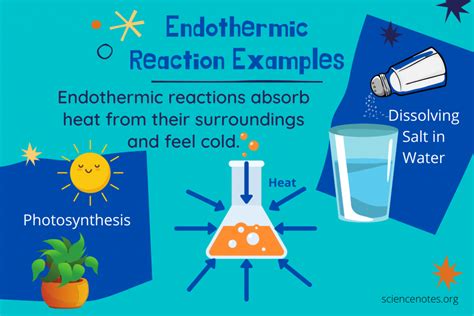
Table of Contents
In an Endothermic Reaction, Energy Is… Absorbed! Understanding Endothermic Processes
Endothermic reactions are a fundamental concept in chemistry, crucial for understanding various natural processes and industrial applications. This comprehensive guide delves deep into the nature of endothermic reactions, exploring their characteristics, examples, and significance. We will unravel the mystery behind the central question: in an endothermic reaction, energy is absorbed. Let's explore this fascinating aspect of chemical thermodynamics.
What is an Endothermic Reaction?
At its core, an endothermic reaction is a chemical reaction where the system absorbs energy from its surroundings. This absorption of energy manifests as a decrease in the temperature of the surroundings. Think of it like this: the reaction needs energy to proceed, and it "pulls" this energy from its environment, making the environment colder. The opposite of an endothermic reaction is an exothermic reaction, where energy is released into the surroundings.
Key Characteristics of Endothermic Reactions:
- Energy Absorption: The most defining characteristic. The reaction requires energy input to occur.
- Temperature Decrease: The surroundings will experience a drop in temperature as energy is absorbed by the reaction.
- Positive Enthalpy Change (ΔH > 0): Enthalpy (ΔH) is a measure of the heat content of a system. A positive value indicates that heat is absorbed.
- Feels Cold: If you were to touch a container undergoing an endothermic reaction, you would often feel a cooling effect.
- Requires an Energy Source: Endothermic reactions often require an external energy source, such as heat, light, or electricity, to initiate and sustain the reaction.
How Does Energy Absorption Work in Endothermic Reactions?
To truly understand how energy is absorbed, we must look at the bonds being broken and formed during the reaction. Endothermic reactions require more energy to break the existing bonds in the reactants than is released when new bonds are formed in the products. This difference in energy is the net energy absorbed from the surroundings.
Imagine it like this: you have a building made of LEGO bricks (reactants). To take it apart (break the bonds), you need to put in effort (energy). Then, you use those same bricks to build something new (products). If the new building requires less effort to construct than the initial effort to deconstruct the old one, the overall process requires a net energy input—an endothermic reaction.
Examples of Endothermic Reactions:
Many everyday processes and industrial applications involve endothermic reactions. Here are some notable examples:
1. Photosynthesis:
This fundamental process in plants is a prime example. Plants absorb sunlight (energy) to convert carbon dioxide and water into glucose (sugar) and oxygen. The energy from the sun is absorbed, making it a classic endothermic reaction.
Equation: 6CO₂ + 6H₂O + Energy (Sunlight) → C₆H₁₂O₆ + 6O₂
2. Melting Ice:
Melting ice cubes require energy from the surroundings to break the hydrogen bonds holding the water molecules together in the solid state. The surroundings lose heat, making the process endothermic.
Equation: H₂O(s) + Energy → H₂O(l)
3. Cooking an Egg:
While cooking an egg seems exothermic (heat is applied), the actual chemical changes occurring within the egg, such as the denaturation of proteins, are endothermic. The heat is absorbed by the proteins, causing them to unfold and change their structure.
4. Dissolving Ammonium Nitrate in Water:
Dissolving ammonium nitrate (NH₄NO₃) in water is a common example demonstrated in chemistry labs. The solution noticeably cools down because the dissolving process absorbs heat from its surroundings.
5. Baking Soda and Vinegar Reaction:
The reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) is often perceived as exothermic due to the gas production. However, the overall reaction is endothermic; the heat absorbed during the reaction is masked by the heat released by the neutralization reaction.
Applications of Endothermic Reactions:
Endothermic reactions, despite their need for energy input, have significant practical applications:
- Refrigeration: Many refrigerants utilize endothermic processes to absorb heat from their surroundings, cooling the environment.
- Instant Cold Packs: These packs commonly used for injuries contain ammonium nitrate and water; when mixed, they create an endothermic reaction, providing a cooling effect.
- Chemical Synthesis: Many industrial processes require energy input to drive specific chemical reactions. Understanding endothermic reactions is crucial for optimizing these processes.
- Climate Control: Photosynthesis plays a vital role in regulating the Earth's temperature by absorbing solar energy.
Understanding Enthalpy Change (ΔH) in Endothermic Reactions:
As mentioned earlier, the enthalpy change (ΔH) is a crucial factor in characterizing endothermic reactions. A positive ΔH value always signifies an endothermic process. The magnitude of ΔH indicates the amount of energy absorbed. A larger positive ΔH indicates a greater energy requirement.
Calculating Enthalpy Change:
Determining the enthalpy change involves measuring the heat absorbed or released during a reaction under constant pressure. Calorimetry is a common experimental technique used for this purpose. The specific heat capacity of the solution and the mass of the reactants are crucial factors in the calculation.
Factors Affecting Endothermic Reactions:
Several factors can influence the rate and extent of an endothermic reaction:
- Temperature: Increasing the temperature generally increases the rate of an endothermic reaction because it provides more energy to overcome the activation energy barrier.
- Concentration: Increasing the concentration of reactants usually increases the rate of the reaction by increasing the frequency of collisions between reactant molecules.
- Surface Area: For reactions involving solids, increasing the surface area of the reactant increases the rate by providing more sites for the reaction to occur.
- Catalyst: A catalyst can lower the activation energy of the reaction, increasing the rate but does not affect the overall enthalpy change.
Distinguishing Endothermic from Exothermic Reactions:
The key difference between endothermic and exothermic reactions lies in their energy exchange with the surroundings:
| Feature | Endothermic Reaction | Exothermic Reaction |
|---|---|---|
| Energy Exchange | Absorbs energy from surroundings | Releases energy to surroundings |
| Temperature | Surroundings cool down | Surroundings heat up |
| Enthalpy Change (ΔH) | Positive (ΔH > 0) | Negative (ΔH < 0) |
| Feels | Cold | Hot |
Conclusion:
In an endothermic reaction, energy is absorbed from the surroundings. This absorption is a fundamental characteristic of these reactions, driving diverse processes from photosynthesis to industrial chemical synthesis. Understanding the principles of endothermic reactions is crucial across various scientific fields and everyday life applications. By grasping the concepts of energy absorption, enthalpy change, and the factors influencing these reactions, we can better appreciate their significant role in shaping our world. This comprehensive exploration has hopefully illuminated the intricate nature of endothermic processes and their widespread implications.
Latest Posts
Latest Posts
-
Find The Inverse Of The Relation
Apr 01, 2025
-
How Are Cellular Respiration And Photosynthesis Related
Apr 01, 2025
-
How Many Rna Polymerases Are Found In Prokaryotes
Apr 01, 2025
-
When Two Parallel Lines Are Crossed By A Transversal
Apr 01, 2025
-
Device That Converts Light Energy Into Electrical Energy
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about In An Endothermic Reaction Energy Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
