How To Find Number Of Moles In A Compound
Juapaving
Mar 23, 2025 · 5 min read
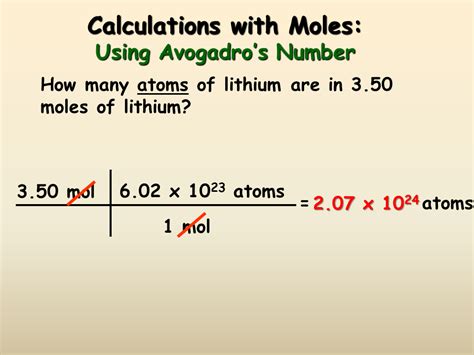
Table of Contents
How to Find the Number of Moles in a Compound: A Comprehensive Guide
Determining the number of moles in a compound is a fundamental concept in chemistry, crucial for various calculations and experiments. Understanding this process allows you to accurately predict reaction yields, determine concentrations, and analyze the composition of substances. This comprehensive guide will walk you through different methods for calculating moles, covering various scenarios and providing practical examples.
Understanding Moles: The Foundation of Chemical Calculations
Before diving into the methods, let's solidify our understanding of what a mole represents. A mole (mol) is a fundamental unit in chemistry, representing Avogadro's number (6.022 x 10<sup>23</sup>) of particles. These particles can be atoms, molecules, ions, or any other specified entity. Think of it like a dozen—a dozen eggs contains 12 eggs, a mole of carbon atoms contains 6.022 x 10<sup>23</sup> carbon atoms. The mole provides a bridge between the macroscopic world (grams, liters) and the microscopic world (atoms, molecules).
Method 1: Using Mass and Molar Mass
This is arguably the most common method for determining the number of moles. It relies on the relationship between mass, molar mass, and the number of moles:
Moles (mol) = Mass (g) / Molar Mass (g/mol)
Molar Mass: This is the mass of one mole of a substance. It's calculated by summing the atomic masses (found on the periodic table) of all atoms in the chemical formula of the compound.
Example 1: Finding moles of water (H₂O)
Let's say we have 18 grams of water. To find the number of moles:
-
Calculate the molar mass of water:
- H: 1.01 g/mol x 2 = 2.02 g/mol
- O: 16.00 g/mol
- Total molar mass = 2.02 g/mol + 16.00 g/mol = 18.02 g/mol
-
Apply the formula:
- Moles = 18 g / 18.02 g/mol ≈ 0.999 moles
Therefore, 18 grams of water contains approximately 1 mole of water molecules.
Example 2: Finding moles of sodium chloride (NaCl)
We have 58.5 grams of NaCl.
-
Calculate the molar mass of NaCl:
- Na: 22.99 g/mol
- Cl: 35.45 g/mol
- Total molar mass = 22.99 g/mol + 35.45 g/mol = 58.44 g/mol
-
Apply the formula:
- Moles = 58.5 g / 58.44 g/mol ≈ 1.001 moles
Approximately 1 mole of NaCl is present in 58.5 grams.
Method 2: Using Volume and Molar Concentration (Molarity)
This method is particularly useful when dealing with solutions. Molarity (M) is defined as the number of moles of solute per liter of solution:
Molarity (M) = Moles (mol) / Volume (L)
To find the number of moles, rearrange the formula:
Moles (mol) = Molarity (M) x Volume (L)
Example 3: Finding moles in a solution
We have 250 mL of a 0.5 M solution of glucose (C₆H₁₂O₆).
-
Convert volume to liters:
- 250 mL = 250 mL x (1 L / 1000 mL) = 0.25 L
-
Apply the formula:
- Moles = 0.5 mol/L x 0.25 L = 0.125 moles
Therefore, there are 0.125 moles of glucose in 250 mL of a 0.5 M solution.
Method 3: Using the Ideal Gas Law (for Gases)
For gaseous compounds, the ideal gas law can be used to determine the number of moles:
PV = nRT
Where:
- P = Pressure (usually in atm)
- V = Volume (usually in Liters)
- n = Number of moles
- R = Ideal gas constant (0.0821 L·atm/mol·K)
- T = Temperature (in Kelvin)
To find the number of moles, rearrange the formula:
n = PV / RT
Example 4: Finding moles of a gas
A gas occupies 5.6 liters at a pressure of 1 atm and a temperature of 273 K.
-
Convert temperature to Kelvin (if not already): 273 K (already in Kelvin)
-
Apply the formula:
- n = (1 atm x 5.6 L) / (0.0821 L·atm/mol·K x 273 K) ≈ 0.25 moles
Therefore, approximately 0.25 moles of gas are present.
Method 4: Using Avogadro's Number and Number of Particles
While less frequently used in practical calculations, this method directly applies Avogadro's number:
Moles (mol) = Number of Particles / Avogadro's Number
Example 5: Finding moles from the number of molecules
We have 3.011 x 10<sup>23</sup> molecules of oxygen (O₂).
- Apply the formula:
- Moles = (3.011 x 10<sup>23</sup> molecules) / (6.022 x 10<sup>23</sup> molecules/mol) = 0.5 moles
Therefore, there are 0.5 moles of oxygen molecules.
Common Mistakes to Avoid
-
Unit Conversion: Ensure all units are consistent before applying any formula. Convert grams to kilograms, milliliters to liters, and Celsius to Kelvin as needed. Inconsistent units will lead to incorrect results.
-
Molar Mass Calculation: Double-check the molar mass calculation to avoid errors in summing atomic masses. Pay close attention to subscripts in chemical formulas.
-
Significant Figures: Report your final answer with the appropriate number of significant figures based on the precision of the input values.
-
Ideal Gas Law Assumptions: Remember the ideal gas law is an approximation. It works best for gases at low pressures and high temperatures. Deviations from ideality can occur under extreme conditions.
Advanced Applications and Considerations
The methods outlined above form the basis for numerous advanced chemical calculations. Understanding moles is essential for:
- Stoichiometry: Calculating the amounts of reactants and products in chemical reactions.
- Titration: Determining the concentration of an unknown solution using a known solution.
- Solution Preparation: Accurately preparing solutions of specific concentrations.
- Gas Law Calculations: Solving problems involving the behavior of gases.
- Thermochemistry: Calculating heat changes in chemical reactions.
Mastering the ability to calculate the number of moles is a cornerstone of chemical understanding. By carefully following the methods described and paying attention to detail, you can confidently tackle various chemical problems and further your understanding of the quantitative aspects of chemistry. Remember to practice regularly with different examples and scenarios to build your proficiency and confidence.
Latest Posts
Latest Posts
-
What Type Of Cell Has Large Vacuoles
Mar 25, 2025
-
Light Microscope And Electron Microscope Differences
Mar 25, 2025
-
A Catalyst Lowers The Activation Energy Of A Reaction By
Mar 25, 2025
-
Whats The Lcm Of 8 And 10
Mar 25, 2025
-
What Is 11 Cm In Inches
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How To Find Number Of Moles In A Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
