How To Find Molarity From Normality
Juapaving
Mar 29, 2025 · 5 min read
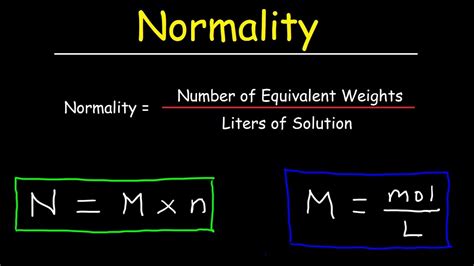
Table of Contents
How to Find Molarity from Normality: A Comprehensive Guide
Understanding the relationship between molarity and normality is crucial in many chemical calculations, particularly in acid-base titrations and other stoichiometric problems. While molarity represents the concentration of a substance in moles per liter, normality focuses on the reactive capacity of a substance. This guide will walk you through the process of calculating molarity from normality, explaining the underlying concepts and providing practical examples.
Understanding Molarity and Normality
Before diving into the conversion, let's solidify our understanding of both terms:
Molarity (M):
Molarity is defined as the number of moles of solute per liter of solution. The formula is:
Molarity (M) = moles of solute / liters of solution
For instance, a 1M solution of sodium chloride (NaCl) contains one mole of NaCl dissolved in one liter of solution.
Normality (N):
Normality is a measure of concentration that takes into account the reactive capacity of a substance. It's defined as the number of equivalents of solute per liter of solution. The key difference lies in the concept of "equivalents".
- Equivalents: The number of equivalents depends on the reaction the substance is involved in. It represents the number of moles of reactive units in one mole of the substance. This is where things get a bit more nuanced.
Determining Equivalents:
The calculation of equivalents varies depending on the type of reaction:
-
Acid-Base Reactions: For acids, the number of equivalents is equal to the number of acidic protons (H⁺) that can be donated per molecule. For example, HCl has one equivalent per mole (monoprotic), H₂SO₄ has two equivalents per mole (diprotic), and H₃PO₄ has three equivalents per mole (triprotic). For bases, the number of equivalents is determined by the number of hydroxide ions (OH⁻) that can be donated per molecule or the number of protons they can accept.
-
Redox Reactions: In redox reactions (reduction-oxidation), the number of equivalents is determined by the number of electrons transferred per mole of the substance. You need to consider the change in oxidation state.
-
Precipitation Reactions: In precipitation reactions, the number of equivalents is determined by the charge of the ion involved in the precipitation.
The Conversion: From Normality to Molarity
The relationship between normality and molarity is straightforward:
Normality (N) = Molarity (M) × n
Where 'n' is the number of equivalents per mole of the solute.
To find molarity from normality, simply rearrange the equation:
Molarity (M) = Normality (N) / n
This means that to convert normality to molarity, you divide the normality by the number of equivalents per mole.
Illustrative Examples:
Let's work through several examples to solidify your understanding:
Example 1: Monoprotic Acid
A solution of hydrochloric acid (HCl) has a normality of 0.5N. Find its molarity.
-
Step 1: Determine the number of equivalents per mole (n). HCl is a monoprotic acid, so n = 1.
-
Step 2: Apply the formula: Molarity (M) = Normality (N) / n = 0.5N / 1 = 0.5M
Therefore, the molarity of the HCl solution is 0.5M.
Example 2: Diprotic Acid
A sulfuric acid (H₂SO₄) solution has a normality of 2N. Calculate its molarity.
-
Step 1: Determine n. H₂SO₄ is a diprotic acid, meaning it has two acidic protons, so n = 2.
-
Step 2: Apply the formula: Molarity (M) = Normality (N) / n = 2N / 2 = 1M
The molarity of the H₂SO₄ solution is 1M.
Example 3: Triprotic Acid
A phosphoric acid (H₃PO₄) solution has a normality of 3N. What is its molarity?
-
Step 1: Determine n. H₃PO₄ is a triprotic acid, so n = 3.
-
Step 2: Apply the formula: Molarity (M) = Normality (N) / n = 3N / 3 = 1M
The molarity of the H₃PO₄ solution is 1M.
Example 4: Base Example
A solution of sodium hydroxide (NaOH) has a normality of 0.1N. Determine its molarity.
-
Step 1: Determine n. NaOH is a monobasic base, meaning it donates one hydroxide ion, so n = 1.
-
Step 2: Apply the formula: Molarity (M) = Normality (N) / n = 0.1N / 1 = 0.1M
The molarity of the NaOH solution is 0.1M.
Example 5: Redox Reaction
Consider a potassium permanganate (KMnO₄) solution used as an oxidizing agent in a redox titration. The normality of the solution is 0.02N. In this specific reaction, KMnO₄ accepts 5 electrons. Determine its molarity.
-
Step 1: Determine n. Since KMnO₄ accepts 5 electrons, n = 5.
-
Step 2: Apply the formula: Molarity (M) = Normality (N) / n = 0.02N / 5 = 0.004M
The molarity of the KMnO₄ solution is 0.004M.
Important Considerations:
-
Context is Crucial: The value of 'n' is entirely dependent on the specific chemical reaction being considered. Always carefully analyze the reaction to determine the number of equivalents.
-
Not Always Applicable: Normality isn't always the most practical measure of concentration. Molarity is generally preferred due to its simpler and more universally applicable nature.
-
Dilution Calculations: Remember that when diluting a solution, the number of moles of solute remains constant. You can use the dilution formula (M₁V₁ = M₂V₂) to calculate the molarity of a diluted solution if you know the initial molarity and volumes. You can also use this relationship with normality (N₁V₁ = N₂V₂).
Conclusion:
Converting normality to molarity is a crucial skill in chemistry, particularly in quantitative analysis. By understanding the concept of equivalents and applying the appropriate formula, you can easily make this conversion and confidently tackle various stoichiometric calculations. Remember that the key is correctly identifying the number of equivalents per mole (n) based on the specific chemical reaction. While normality might seem more complex than molarity, mastering this conversion greatly expands your capabilities in solving chemical problems. Always carefully examine the context of the problem to correctly determine the value of 'n' and perform the calculation accurately. With consistent practice, you will become proficient in this important conversion and its application in various chemical situations.
Latest Posts
Latest Posts
-
A Cell Placed In A Hypotonic Solution Will
Mar 31, 2025
-
How Does An Amoeba Get Its Food
Mar 31, 2025
-
What Is The Prime Factorization Of 350
Mar 31, 2025
-
Is Calcium Hydroxide An Acid Or A Base
Mar 31, 2025
-
Greatest Common Factor 36 And 90
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How To Find Molarity From Normality . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
