A Cell Placed In A Hypotonic Solution Will
Juapaving
Mar 31, 2025 · 6 min read
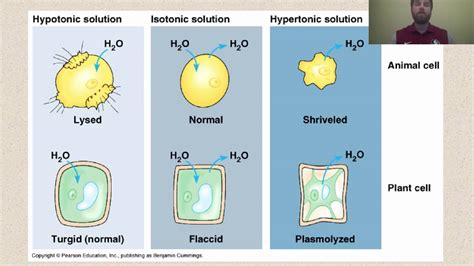
Table of Contents
A Cell Placed in a Hypotonic Solution Will… Undergo Osmosis and Potential Lysis
Understanding how cells behave in different environments is crucial in biology. One key concept is the effect of tonicity, specifically what happens when a cell is placed in a hypotonic solution. This article delves deep into the process, exploring the underlying mechanisms, the consequences for various cell types, and the applications of this knowledge in various fields.
What is a Hypotonic Solution?
Before exploring the effects, let's define our terms. A hypotonic solution is one that has a lower solute concentration compared to another solution, often a cell's internal environment (cytoplasm). Solute concentration refers to the amount of dissolved substances (like salts, sugars, proteins) present in a solution. The opposite of a hypotonic solution is a hypertonic solution, which has a higher solute concentration. An isotonic solution has an equal solute concentration.
The movement of water across a selectively permeable membrane, driven by differences in solute concentration, is called osmosis. Water always moves from a region of high water concentration (low solute concentration) to a region of low water concentration (high solute concentration).
Osmosis: The Driving Force
When a cell is placed in a hypotonic solution, the concentration of solutes inside the cell is higher than in the surrounding solution. Consequently, the water concentration outside the cell is higher. This difference in water concentration drives osmosis: water rushes into the cell across the cell membrane to equalize the concentration gradient.
Think of it like this: imagine a balloon (representing the cell) filled with a concentrated sugar solution. If you place this balloon in a beaker of pure water, the water will move into the balloon, causing it to swell. This is analogous to what happens to a cell in a hypototonic solution.
The Role of the Cell Membrane
The cell membrane plays a crucial role in this process. It is selectively permeable, meaning it allows some substances to pass through while restricting others. Water molecules, being small and uncharged, can easily pass through the membrane via osmosis, but larger solute molecules generally cannot. This selective permeability is vital for maintaining the cell's internal environment.
Consequences for the Cell: Swelling and Lysis
The influx of water into the cell causes it to swell. The extent of swelling depends on several factors, including the initial solute concentration difference, the cell's membrane elasticity, and the cell wall's presence (if applicable).
For animal cells, which lack a rigid cell wall, prolonged exposure to a hypotonic solution can lead to lysis, or cell bursting. The cell membrane can only stretch to a certain point before it ruptures, releasing the cell's contents. This is a catastrophic event, leading to cell death.
Plant cells, on the other hand, have a rigid cell wall surrounding the cell membrane. The cell wall provides structural support and prevents excessive swelling. When a plant cell is placed in a hypotonic solution, it initially swells, but the cell wall resists further expansion. This results in turgor pressure, the pressure exerted by the cell contents against the cell wall. Turgor pressure is essential for maintaining the plant's rigidity and shape; a plant wilts when its cells lose turgor pressure.
Types of Cells and Their Responses
Different types of cells respond differently to hypotonic solutions based on their inherent properties and the specific environment.
Animal Cells: Vulnerability to Lysis
Animal cells, lacking a protective cell wall, are particularly susceptible to lysis in hypotonic solutions. Red blood cells (erythrocytes), for instance, are easily lysed in hypotonic solutions, a process known as hemolysis. This is why intravenous fluids administered to patients are carefully formulated to be isotonic, preventing hemolysis and other harmful effects.
Plant Cells: Turgor Pressure and Plasmolysis
Plant cells display a more complex response. As mentioned earlier, they develop turgor pressure in hypotonic solutions. However, if the plant cell is placed in a hypertonic solution, water will move out of the cell, causing the cell membrane to pull away from the cell wall—a process called plasmolysis. Plasmolysis leads to wilting and ultimately, cell death if prolonged.
Bacterial Cells: Cell Wall Protection and Osmotic Pressure Regulation
Bacterial cells, like plant cells, possess a cell wall that protects them from lysis in hypotonic solutions. However, the bacterial cell wall's composition and structure differ from plant cell walls, influencing their response to osmotic stress. Bacteria also employ various strategies to regulate osmotic pressure, including the synthesis of compatible solutes that help balance the internal and external osmotic pressure.
Applications of Hypotonic Solutions
The understanding of how cells behave in hypotonic solutions has far-reaching applications in various fields:
-
Medicine: Intravenous fluids need to be isotonic to prevent hemolysis. However, hypotonic solutions can be used in specific medical procedures, such as hydrating dehydrated patients or aiding in certain drug delivery systems.
-
Agriculture: Understanding osmosis is crucial in plant physiology, influencing irrigation techniques and fertilizer application. Maintaining appropriate osmotic pressure in the soil solution helps ensure optimal plant growth and yield.
-
Food science: The preservation of food often involves manipulating osmotic pressure. Pickling, for example, uses a hypertonic solution to draw water out of microorganisms, preventing their growth and spoilage.
-
Biotechnology: In cell culture, maintaining the correct tonicity of the culture medium is essential for healthy cell growth and function. Researchers often use hypotonic and isotonic solutions to manipulate cell processes and perform experiments.
Factors Affecting Osmosis Rate
Several factors can influence the rate at which osmosis occurs:
-
Concentration gradient: The steeper the concentration gradient (the greater the difference in solute concentration between the two solutions), the faster the rate of osmosis.
-
Temperature: Higher temperatures increase the kinetic energy of water molecules, leading to a faster rate of osmosis.
-
Membrane permeability: The more permeable the membrane to water, the faster the osmosis.
-
Surface area: A larger surface area of the membrane increases the rate of osmosis.
Conclusion: A Dynamic Equilibrium
When a cell is placed in a hypotonic solution, water moves into the cell via osmosis. This process, while essential for maintaining cell turgor and function in some cases, can also lead to cell lysis if not carefully regulated. Understanding the intricacies of osmosis and the response of different cell types is paramount in diverse fields ranging from medicine and agriculture to biotechnology and food science. The dynamic interplay between the cell's internal environment and the external solution highlights the remarkable adaptation of cells to osmotic challenges and underscores the importance of maintaining an appropriate osmotic balance for healthy cell function. The continued research in this area will continue to reveal more nuanced details of this fundamental biological process and its broader implications.
Latest Posts
Latest Posts
-
The First Organisms On Earth Were
Apr 02, 2025
-
Whats The Prime Factorization Of 12
Apr 02, 2025
-
The Property Of Letting Light Pass Through Something
Apr 02, 2025
-
What Are The Greatest Common Factors Of 75
Apr 02, 2025
-
Do Parallelograms Have 4 Equal Sides
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about A Cell Placed In A Hypotonic Solution Will . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
