How Much Valence Electrons Does Oxygen Have
Juapaving
Mar 29, 2025 · 6 min read
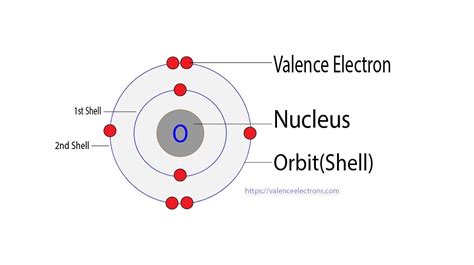
Table of Contents
How Many Valence Electrons Does Oxygen Have? A Deep Dive into Atomic Structure and Bonding
Oxygen, a vital element for life as we know it, plays a crucial role in numerous biological and chemical processes. Understanding its atomic structure, particularly the number of valence electrons, is key to grasping its reactivity and the diverse compounds it forms. This comprehensive article will delve into the fascinating world of oxygen's valence electrons, exploring its electronic configuration, bonding behavior, and significance in various contexts.
Understanding Valence Electrons: The Key to Chemical Reactivity
Before we dive into oxygen specifically, let's establish a fundamental understanding of valence electrons. These are the electrons located in the outermost shell, or energy level, of an atom. They are the electrons most involved in chemical bonding, determining an element's reactivity and the types of bonds it can form. The number of valence electrons dictates how many bonds an atom can typically make.
The outermost shell is also known as the valence shell. Atoms strive for stability, often achieved by having a full valence shell. This principle is the foundation of the octet rule, stating that atoms tend to gain, lose, or share electrons to achieve eight electrons in their valence shell (except for hydrogen and helium, which aim for two electrons).
Oxygen's Electronic Configuration: Unveiling the Valence Electrons
Oxygen's atomic number is 8, meaning it possesses 8 protons and 8 electrons in a neutral atom. To determine the number of valence electrons, we need to examine its electronic configuration. This describes how electrons are distributed among the different energy levels or shells within the atom.
Oxygen's electronic configuration is 1s²2s²2p⁴. Let's break this down:
- 1s²: Two electrons occupy the first energy level (n=1), specifically the s subshell.
- 2s²: Two electrons occupy the second energy level (n=2), in the s subshell.
- 2p⁴: Four electrons occupy the second energy level's p subshell. The p subshell can hold a maximum of six electrons.
The valence electrons are those in the outermost shell, which, in oxygen's case, is the second energy level (n=2). Therefore, oxygen has six valence electrons (2 from the 2s subshell and 4 from the 2p subshell).
Visualizing Oxygen's Electron Arrangement: The Bohr Model
The Bohr model, while a simplified representation, provides a useful visualization of electron arrangement. It depicts electrons orbiting the nucleus in specific energy levels or shells. For oxygen, you'd see two electrons in the first shell and six electrons in the second shell. These six electrons in the second shell are the valence electrons.
Oxygen's Bonding Behavior: A Consequence of Six Valence Electrons
Oxygen's six valence electrons significantly influence its chemical behavior. To achieve a stable octet, oxygen needs to gain two more electrons. This tendency leads to the formation of two covalent bonds or the acceptance of two electrons to form an ionic bond.
Covalent Bonding in Oxygen: Sharing Electrons for Stability
Covalent bonds involve the sharing of electrons between atoms. Oxygen frequently forms covalent bonds with other atoms, especially hydrogen, carbon, and other nonmetals. A classic example is the oxygen molecule (O₂), where two oxygen atoms share two pairs of electrons, forming a double bond. Each oxygen atom effectively gains eight electrons in its valence shell.
Other examples of covalent bonding involving oxygen include:
- Water (H₂O): Oxygen forms two single covalent bonds with two hydrogen atoms.
- Carbon dioxide (CO₂): Oxygen forms two double covalent bonds with a single carbon atom.
- Organic molecules: Oxygen is a key component of numerous organic molecules, forming various covalent bonds with carbon, hydrogen, and other atoms.
Ionic Bonding with Oxygen: Gaining Electrons to Form Ions
Ionic bonds involve the transfer of electrons between atoms. Oxygen's high electronegativity (its tendency to attract electrons) means it readily accepts electrons from other atoms, particularly metals. This electron transfer creates ions: a negatively charged oxygen ion (oxide ion, O²⁻) and a positively charged metal ion.
Examples of ionic compounds containing oxygen include:
- Magnesium oxide (MgO): Magnesium donates two electrons to oxygen, forming Mg²⁺ and O²⁻ ions.
- Sodium oxide (Na₂O): Each sodium atom donates one electron to oxygen, resulting in Na⁺ and O²⁻ ions.
- Many metal oxides: Oxygen readily forms ionic compounds with a vast array of metals.
The Significance of Oxygen's Valence Electrons: Biological and Chemical Importance
The presence of six valence electrons in oxygen is not merely an abstract concept; it directly relates to oxygen's crucial role in numerous processes:
Respiration: Oxygen's Vital Role in Energy Production
Cellular respiration, the process by which organisms generate energy, relies heavily on oxygen's ability to accept electrons. During respiration, oxygen acts as the final electron acceptor in the electron transport chain, facilitating the production of ATP (adenosine triphosphate), the primary energy currency of cells. Without oxygen’s capacity to accept electrons due to its six valence electrons, this critical energy production pathway would be impossible.
Oxidation and Reduction Reactions (Redox Reactions): Oxygen as an Oxidizing Agent
Oxygen's high electronegativity makes it a powerful oxidizing agent. It readily accepts electrons from other substances, causing them to become oxidized (lose electrons). This property is fundamental to many chemical reactions, including combustion and corrosion. The six valence electrons are the driving force behind oxygen's oxidizing ability.
Oxygen in the Environment: Ozone Layer and Atmospheric Chemistry
Oxygen's role in the environment is equally significant. Ozone (O₃), a molecule consisting of three oxygen atoms, forms a protective layer in the stratosphere, shielding the Earth from harmful ultraviolet (UV) radiation. Understanding the bonding and reactivity of oxygen is crucial for studying atmospheric chemistry and environmental processes.
Conclusion: The Importance of Understanding Oxygen's Valence Electrons
The seemingly simple question of how many valence electrons oxygen possesses opens a door to a vast understanding of its chemical behavior, its biological importance, and its impact on the world around us. The six valence electrons dictate oxygen's reactivity, its ability to form covalent and ionic bonds, and its role in vital processes like respiration and oxidation-reduction reactions. This knowledge is fundamental to many scientific fields, from biology and chemistry to environmental science and materials science. By grasping the significance of oxygen's valence electrons, we gain a deeper appreciation for the fundamental principles of chemistry and the vital role this element plays in sustaining life on Earth. Further research into the specific properties and reactions of oxygen continues to reveal the multifaceted nature of this essential element, emphasizing the ongoing importance of understanding its atomic structure and bonding behavior. Therefore, the seemingly simple answer – six valence electrons – unlocks a complex world of chemical and biological processes.
Latest Posts
Latest Posts
-
What Is The Difference Between An Ecosystem And An Environment
Mar 31, 2025
-
What Are The Prime Numbers Between 20 And 30
Mar 31, 2025
-
What Is 30 As A Decimal
Mar 31, 2025
-
Write The Prime Factorization Of 66
Mar 31, 2025
-
What Is 3 20 As A Percent
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Much Valence Electrons Does Oxygen Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
