How Many Valence Electrons Do Carbon Have
Juapaving
Mar 29, 2025 · 6 min read
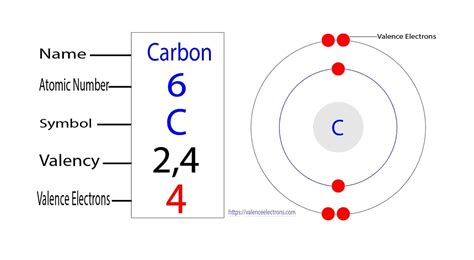
Table of Contents
How Many Valence Electrons Does Carbon Have? Understanding Carbon's Bonding Power
Carbon. The very word conjures images of diamonds, graphite, and the foundation of all known life. This remarkable element's unique properties stem from a seemingly simple fact: it has four valence electrons. This seemingly small number is the key to understanding carbon's incredible versatility and its crucial role in chemistry and biology. This article will delve deep into the significance of carbon's four valence electrons, exploring its bonding capabilities, the resulting diverse structures, and the implications for organic chemistry and beyond.
Understanding Valence Electrons: The Key to Chemical Bonding
Before diving into the specifics of carbon, let's establish a solid understanding of valence electrons. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the most loosely bound and, therefore, the most likely to participate in chemical bonding. The number of valence electrons determines an atom's reactivity and the types of bonds it can form. Atoms tend to react in ways that achieve a stable electron configuration, often resembling the noble gases with their full outermost shells.
Carbon's Electronic Configuration: The Foundation of its Reactivity
Carbon's atomic number is 6, meaning it has six protons and six electrons. Its electronic configuration is 1s²2s²2p². This configuration reveals that:
- Two electrons occupy the first energy level (1s orbital).
- Four electrons occupy the second energy level (2s and 2p orbitals). These are the valence electrons.
It's these four valence electrons that are responsible for carbon's exceptional ability to form a vast array of molecules. The significance of this number cannot be overstated. It allows carbon to:
-
Form four covalent bonds: Carbon readily shares its four valence electrons with other atoms, creating strong and stable covalent bonds. This is crucial for the formation of long chains, rings, and complex three-dimensional structures.
-
Exhibit diverse bonding geometries: The four valence electrons can be arranged in various spatial arrangements, leading to different molecular geometries such as tetrahedral, trigonal planar, and linear. This versatility is essential for the complexity and diversity of organic molecules.
Carbon's Bonding Prowess: Exploring Covalent Bonds and Hybrid Orbitals
Carbon's ability to form four covalent bonds is a direct consequence of its four valence electrons. A covalent bond is a chemical bond formed by the sharing of electron pairs between atoms. In carbon, this sharing allows it to achieve a stable octet, resembling the noble gas neon.
The formation of covalent bonds in carbon isn't simply a matter of straightforward sharing; it involves the concept of hybrid orbitals. To understand this, let's consider the energy levels:
- The 2s orbital: This orbital contains two electrons.
- The 2p orbitals: These three orbitals (2px, 2py, 2pz) each contain one electron.
In many carbon-containing compounds, these orbitals hybridize to form four equivalent sp³ hybrid orbitals. This hybridization explains the tetrahedral geometry observed in many organic molecules like methane (CH₄). Each sp³ hybrid orbital contains one electron and participates in the formation of a single covalent bond with another atom.
However, other hybridization schemes are possible depending on the bonding situation:
-
sp² hybridization: In this case, one 2s orbital and two 2p orbitals hybridize to form three sp² hybrid orbitals, leaving one 2p orbital unhybridized. This results in a trigonal planar geometry, often seen in molecules like ethene (C₂H₄).
-
sp hybridization: Here, one 2s orbital and one 2p orbital hybridize to form two sp hybrid orbitals, leaving two 2p orbitals unhybridized. This leads to a linear geometry observed in molecules like ethyne (C₂H₂).
These different hybridization schemes significantly contribute to the vast structural diversity of carbon-based compounds.
The Significance of Carbon's Four Valence Electrons: A Macro View
The seemingly simple fact that carbon possesses four valence electrons has profound implications across diverse scientific fields:
Organic Chemistry: The Foundation of Life
Organic chemistry, the study of carbon-containing compounds, is fundamentally built upon carbon's ability to form strong, stable, and diverse bonds. The vast array of organic molecules, from simple hydrocarbons to complex biomolecules like proteins and DNA, owe their existence to carbon's unique bonding characteristics. The four valence electrons allow for the creation of long chains, branched structures, rings, and complex three-dimensional architectures, providing the structural basis for life itself.
Materials Science: From Diamonds to Graphene
Carbon's ability to form different types of bonds also has immense importance in materials science. The strong covalent bonds in diamond lead to its exceptional hardness and high refractive index. In contrast, the layered structure of graphite, with its weaker interlayer bonding, results in its softness and ability to conduct electricity. More recently, the discovery of graphene, a single layer of graphite, has revolutionized materials science due to its remarkable strength, conductivity, and potential applications in electronics and other fields. These diverse properties all stem from carbon's fundamental ability to bond in multiple ways.
Nanotechnology: Exploring the Potential of Carbon Nanotubes
Carbon nanotubes, cylindrical structures formed from rolled-up graphene sheets, represent another exciting area where carbon's unique properties are harnessed. Their high tensile strength, electrical conductivity, and unique mechanical properties make them promising materials for various applications, including electronics, composites, and drug delivery systems. Again, the foundation for these remarkable materials is carbon's four valence electrons and its ability to form strong covalent bonds.
Carbon's Role in Biological Systems: Life's Building Block
The biological significance of carbon cannot be overstated. It is the backbone of all known life forms. The four valence electrons enable carbon to form the long chains, rings, and complex three-dimensional structures necessary for the creation of:
-
Carbohydrates: These essential molecules provide energy and structural support to living organisms. Their structures are based on chains of carbon atoms bonded to oxygen and hydrogen.
-
Lipids: Fats, oils, and waxes are examples of lipids, crucial for energy storage, cell membrane structure, and hormone production. Their structures frequently involve long hydrocarbon chains.
-
Proteins: Proteins are vital for numerous cellular functions, including catalysis, transport, and structural support. Their structures are based on amino acids, which contain carbon atoms in their backbones.
-
Nucleic acids (DNA and RNA): These molecules carry the genetic information of living organisms. Their structures are based on nucleotides, which incorporate carbon atoms in their sugar and base components.
The versatility of carbon in forming diverse bonds makes it the ideal element for the construction of these complex biological macromolecules. Without carbon's four valence electrons, life as we know it would be impossible.
Conclusion: The Unsurpassed Versatility of Carbon
In conclusion, the simple fact that carbon has four valence electrons is the key to unlocking its extraordinary versatility and its fundamental role in chemistry and biology. This number allows it to form strong and stable covalent bonds, exhibit diverse bonding geometries, and construct a vast array of molecules with varying complexities and functionalities. From the hard diamonds to the soft graphite, from the simple methane molecule to the complex biomolecules that underpin life, carbon's four valence electrons underpin the rich diversity and remarkable properties we see in the world around us. The continued exploration of carbon's bonding capabilities promises to lead to further breakthroughs in materials science, nanotechnology, and our understanding of the fundamental building blocks of life itself.
Latest Posts
Latest Posts
-
What Is 15 Percent Of 250
Apr 01, 2025
-
Temperature And Kinetic Energy Are Proportional
Apr 01, 2025
-
What 2 Subatomic Particles Make Up The Nucleus
Apr 01, 2025
-
How Many Inches Are 50 Cm
Apr 01, 2025
-
What Is The Lcm Of 4 And 5
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Do Carbon Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
