Temperature And Kinetic Energy Are ___________ Proportional.
Juapaving
Apr 01, 2025 · 6 min read
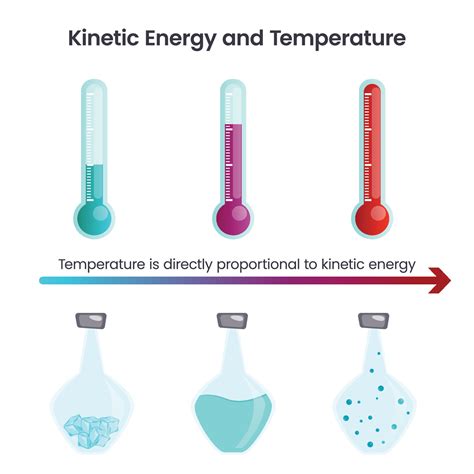
Table of Contents
Temperature and Kinetic Energy Are Directly Proportional
The relationship between temperature and kinetic energy is fundamental to our understanding of thermodynamics and the behavior of matter. In short, temperature and kinetic energy are directly proportional. This means that as temperature increases, the average kinetic energy of the particles within a substance also increases, and vice versa. This seemingly simple statement underpins a vast array of phenomena, from the expansion of gases to the melting of solids. Let's delve deeper into this crucial relationship, exploring the underlying principles, its implications, and some real-world examples.
Understanding Kinetic Energy
Before we explore the relationship, let's define kinetic energy. Kinetic energy is the energy an object possesses due to its motion. For microscopic particles like atoms and molecules, kinetic energy manifests as vibrational, rotational, and translational motion.
- Translational motion: This refers to the movement of particles from one location to another. Think of a gas molecule bouncing around in a container.
- Rotational motion: This involves the spinning of particles around their own axes. Molecules with complex structures exhibit significant rotational kinetic energy.
- Vibrational motion: This refers to the oscillations of atoms within a molecule or the vibrations of atoms in a solid lattice.
The total kinetic energy of a system is the sum of the kinetic energies of all its constituent particles. It's important to remember that we're usually dealing with the average kinetic energy of the particles, as their individual energies will vary constantly due to collisions and interactions.
The Direct Proportionality: Temperature as a Measure of Average Kinetic Energy
Temperature, at a macroscopic level, is a measure of the average kinetic energy of the particles in a system. The higher the temperature, the higher the average kinetic energy of the particles. This is a crucial point: temperature doesn't measure the total kinetic energy, but rather the average kinetic energy. A large, cold object can have a higher total kinetic energy than a small, hot object, but the average kinetic energy of the particles in the hot object will be significantly greater.
This direct proportionality is best described mathematically as:
KE ∝ T
where:
- KE represents the average kinetic energy of the particles.
- T represents the absolute temperature (usually measured in Kelvin).
The proportionality constant depends on the nature of the particles (e.g., their mass) and the number of particles in the system. A more precise equation, applicable to monatomic ideal gases, is:
KE = (3/2) kT
where:
- k is the Boltzmann constant (a fundamental constant in physics).
This equation highlights the direct proportionality between average kinetic energy and absolute temperature. Note that the use of Kelvin is essential; Kelvin is an absolute temperature scale where 0 K represents the absolute absence of kinetic energy. Using Celsius or Fahrenheit would introduce an offset and invalidate the direct proportionality.
Implications of the Direct Proportionality
The direct proportionality between temperature and kinetic energy has far-reaching consequences across various scientific disciplines:
1. States of Matter:
The relationship explains the different states of matter. At low temperatures, the average kinetic energy is low, leading to strong intermolecular forces dominating. This results in solids, where particles are tightly bound. As temperature increases, the kinetic energy rises, overcoming intermolecular forces, leading to liquids and eventually gases where particles move more freely.
2. Thermal Expansion:
When the temperature of a substance increases, the average kinetic energy of its particles increases. This leads to increased particle movement and collisions, resulting in an increase in the average distance between particles. This is manifested as thermal expansion, where materials expand in volume as temperature increases.
3. Gas Laws:
The ideal gas law, PV = nRT, is a direct consequence of the relationship between temperature and kinetic energy. The pressure (P) exerted by a gas is directly related to the frequency and force of collisions between gas particles and the container walls. Higher temperature means higher kinetic energy, leading to more frequent and forceful collisions and thus higher pressure.
4. Diffusion and Effusion:
The rate at which gases diffuse (spread out) or effuse (escape through a small hole) is directly related to their average kinetic energy. Higher temperature means higher kinetic energy, leading to faster diffusion and effusion rates.
5. Chemical Reactions:
Temperature influences the rate of chemical reactions. Higher temperatures mean higher kinetic energy, leading to more frequent and energetic collisions between reactant molecules, increasing the probability of successful reactions. This is why many chemical reactions proceed faster at higher temperatures.
Real-World Examples
The direct proportionality between temperature and kinetic energy is observable in numerous everyday phenomena:
- Heating a balloon: When you heat a balloon, the air molecules inside gain kinetic energy. They move faster and collide more frequently with the balloon's walls, causing the balloon to expand.
- Boiling water: As water is heated, its molecules gain kinetic energy. At 100°C (at standard atmospheric pressure), the kinetic energy becomes sufficient to overcome intermolecular forces, leading to the transition to the gaseous state (boiling).
- Melting ice: Heating ice increases the kinetic energy of water molecules. At 0°C (at standard atmospheric pressure), the kinetic energy becomes sufficient to overcome the rigid structure of the ice lattice, leading to melting.
- Cooking food: Cooking involves transferring heat energy to food, increasing the kinetic energy of the molecules within the food. This leads to changes in texture and chemical reactions, making the food palatable.
Exceptions and Limitations
While the direct proportionality between temperature and kinetic energy is a powerful and widely applicable concept, it's essential to acknowledge its limitations:
- Non-ideal gases: The equation KE = (3/2)kT is accurate only for ideal gases, which are theoretical constructs. Real gases deviate from ideal behavior, especially at high pressures and low temperatures, where intermolecular forces become significant.
- Complex molecules: For complex molecules with multiple atoms, the relationship becomes more nuanced as rotational and vibrational energies contribute significantly to the overall energy.
- Quantum effects: At very low temperatures, close to absolute zero, quantum effects become significant, and classical mechanics (on which the direct proportionality is based) breaks down. The concept of temperature itself needs reinterpretation at these scales.
Conclusion
The direct proportionality between temperature and kinetic energy is a cornerstone of thermodynamics and physical chemistry. Understanding this relationship is crucial for comprehending a wide range of phenomena, from the behavior of gases to the states of matter and the rates of chemical reactions. Although there are exceptions and limitations, particularly at extreme conditions, the direct proportionality provides a robust framework for understanding the behavior of matter at a molecular level and explaining countless observations in the macroscopic world. This fundamental connection is a testament to the elegance and power of physics in explaining the seemingly complex world around us. Further investigation into this relationship continues to refine our models and deepen our understanding of the physical world. Continual research in areas like nanotechnology and materials science hinges on a thorough grasp of the principles discussed in this article, highlighting the ongoing relevance and importance of the direct proportionality between temperature and kinetic energy.
Latest Posts
Latest Posts
-
What Is The Difference Between A Niche And A Habitat
Apr 02, 2025
-
What Is 30 Percent Of 120
Apr 02, 2025
-
Difference Between Violet And Purple Color
Apr 02, 2025
-
How Many Mm Is 3 Cm
Apr 02, 2025
-
A Red Blood Cell Placed In A Hypertonic Medium Will
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Temperature And Kinetic Energy Are ___________ Proportional. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
