What 2 Subatomic Particles Make Up The Nucleus
Juapaving
Apr 01, 2025 · 6 min read
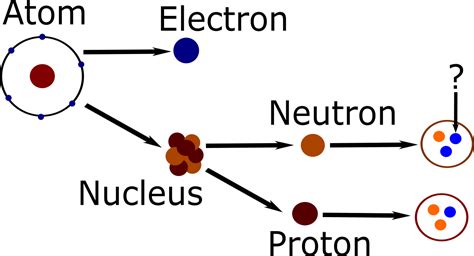
Table of Contents
What Two Subatomic Particles Make Up the Nucleus? A Deep Dive into Protons and Neutrons
The atom, once considered the indivisible building block of matter, has been revealed through centuries of scientific inquiry to be a complex system of even smaller constituents. At the heart of this system lies the nucleus, a tiny, dense core containing the majority of the atom's mass. But what exactly is the nucleus made of? The answer, surprisingly simple yet profoundly significant, is protons and neutrons. These two subatomic particles, collectively known as nucleons, are the fundamental components that determine an atom's identity and behavior. This article delves deep into the properties, characteristics, and interactions of protons and neutrons, exploring their role in shaping the world around us.
Understanding Protons: The Positively Charged Core
Protons, denoted by the symbol 'p' or 'p⁺', are positively charged particles. This positive charge is crucial to the structure of the atom, as it dictates the electrostatic interactions that bind electrons to the nucleus. Each proton carries a single unit of positive charge, equal in magnitude but opposite in sign to the charge of an electron. This fundamental charge is a cornerstone of physics and underlies many electrical phenomena.
Properties of Protons:
-
Mass: Protons possess a relatively large mass compared to electrons. Their mass is approximately 1836 times that of an electron. This significant mass difference contributes substantially to the overall mass of the atom, as the nucleus concentrates almost all of the atomic mass. This mass is approximately 1.6726 × 10⁻²⁷ kg.
-
Charge: As previously mentioned, protons carry a single unit of positive electric charge (+1e), where 'e' represents the elementary charge. This positive charge is responsible for the attractive force between protons and negatively charged electrons, holding the atom together.
-
Spin: Protons are fermions, meaning they possess a half-integer spin (1/2 in units of ħ, the reduced Planck constant). This intrinsic angular momentum contributes to the atom's overall magnetic properties and plays a vital role in nuclear interactions.
-
Composition: Protons are not fundamental particles; they are composed of even smaller constituents called quarks. Specifically, a proton comprises three quarks: two up quarks and one down quark. These quarks are bound together by the strong nuclear force, mediated by gluons.
-
Stability: Protons are remarkably stable particles. While theoretically possible, proton decay has never been observed, and its half-life, if it exists, is predicted to be extraordinarily long, far exceeding the age of the universe. This stability is essential for the stability of atoms and the structure of matter.
Delving into Neutrons: The Neutral Counterparts
Neutrons, symbolized as 'n' or 'n⁰', are neutral particles, carrying no net electric charge. This neutrality plays a crucial role in nuclear stability. Unlike protons, which repel each other due to their positive charges, neutrons contribute to the strong nuclear force without adding to the electrostatic repulsion within the nucleus.
Properties of Neutrons:
-
Mass: Neutrons are slightly more massive than protons, with a mass of approximately 1.6749 × 10⁻²⁷ kg. This small mass difference is significant in certain nuclear processes.
-
Charge: As their name suggests, neutrons have no net electric charge (0e). Their neutrality allows them to exist within the nucleus without significantly contributing to the electrostatic repulsion between protons.
-
Spin: Similar to protons, neutrons are fermions with a half-integer spin (1/2). Their spin contributes to the atom's overall magnetic moment and participates in nuclear interactions.
-
Composition: Like protons, neutrons are also composed of three quarks: one up quark and two down quarks. The difference in quark composition between protons and neutrons accounts for their slight mass difference.
-
Stability: Free neutrons are unstable and decay through beta decay into a proton, an electron, and an antineutrino. This decay process has a half-life of approximately 10 minutes. However, neutrons bound within a stable nucleus are typically stable, their decay prevented by the strong nuclear force.
The Strong Nuclear Force: The Glue Holding the Nucleus Together
The sheer proximity of positively charged protons within the nucleus raises a fundamental question: why don't they repel each other and cause the nucleus to fly apart? The answer lies in the strong nuclear force, one of the four fundamental forces in nature. This force is significantly stronger than the electromagnetic force at short distances, overcoming the electrostatic repulsion between protons and binding both protons and neutrons together within the nucleus.
Characteristics of the Strong Nuclear Force:
-
Short Range: The strong nuclear force is effective only over extremely short distances, on the order of the size of the nucleus itself. Beyond this range, its influence becomes negligible.
-
Attractive Force: It acts as an attractive force between nucleons, binding them together within the nucleus. This attractive force is responsible for holding the nucleus together, despite the electrostatic repulsion between protons.
-
Saturation: The strong nuclear force exhibits a property called saturation, meaning that a nucleon interacts strongly only with a limited number of its nearest neighbors. This property limits the size of stable nuclei.
Isotopes and the Role of Neutrons in Nuclear Stability
The number of protons in an atom's nucleus defines its atomic number and determines its chemical element. However, the number of neutrons can vary for a given element, leading to the existence of isotopes. Isotopes are atoms of the same element (same number of protons) but with different numbers of neutrons.
Some isotopes are stable, while others are radioactive, meaning they undergo spontaneous decay. The neutron-to-proton ratio plays a crucial role in determining the stability of an isotope. A balance between the strong nuclear force (which favors more neutrons for stability in heavier nuclei) and the electrostatic repulsion between protons is crucial for nuclear stability. Too few neutrons, and the electrostatic repulsion will dominate, leading to instability. Too many neutrons, and the nucleus may become unstable due to neutron-rich conditions.
The Significance of Protons and Neutrons: A Broader Perspective
The study of protons and neutrons has far-reaching implications across many scientific disciplines. Their properties and interactions are central to understanding:
-
Nuclear Physics: The field of nuclear physics relies heavily on understanding the structure and behavior of the nucleus, which is directly linked to the properties of protons and neutrons. This understanding is crucial for applications like nuclear energy and nuclear medicine.
-
Particle Physics: Protons and neutrons are themselves composed of quarks, highlighting the deeper layers of matter's structure investigated in particle physics. Research in this area aims to uncover the fundamental building blocks of matter and their interactions.
-
Astrophysics and Cosmology: The abundance of elements in the universe is governed by nuclear processes in stars and supernovae. Understanding the role of protons and neutrons in these processes is vital for understanding the evolution of the universe and the formation of elements.
-
Material Science: The properties of materials are directly related to the atomic structure, which is fundamentally determined by the nucleus' composition of protons and neutrons. Understanding these relationships can lead to the design of new materials with tailored properties.
Conclusion: A Foundation of Matter
In conclusion, protons and neutrons are the fundamental building blocks of the atomic nucleus. Their properties—mass, charge, and spin—along with the strong nuclear force that binds them together, determine the stability and behavior of atomic nuclei. A deep understanding of these subatomic particles is essential for advancing our knowledge across various fields of science, from nuclear physics to cosmology. Further research continues to refine our understanding of their intricate interactions and their role in shaping the world around us. The journey into the subatomic realm remains an ongoing quest, revealing ever-more complex and fascinating aspects of matter's fundamental nature.
Latest Posts
Latest Posts
-
What Is The Difference Between A Niche And A Habitat
Apr 02, 2025
-
What Is 30 Percent Of 120
Apr 02, 2025
-
Difference Between Violet And Purple Color
Apr 02, 2025
-
How Many Mm Is 3 Cm
Apr 02, 2025
-
A Red Blood Cell Placed In A Hypertonic Medium Will
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What 2 Subatomic Particles Make Up The Nucleus . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
