How Many Protons Neutrons And Electrons In Sodium
Juapaving
Mar 18, 2025 · 6 min read
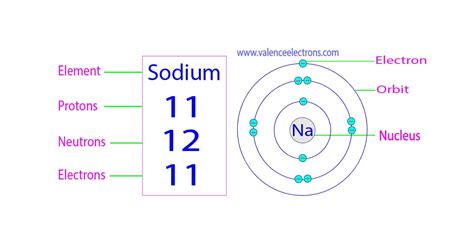
Table of Contents
How Many Protons, Neutrons, and Electrons in Sodium? A Deep Dive into Atomic Structure
Sodium, a ubiquitous element essential for life, plays a crucial role in various biological processes and industrial applications. Understanding its atomic structure, particularly the number of protons, neutrons, and electrons, is fundamental to comprehending its chemical behavior and properties. This comprehensive guide will explore sodium's atomic composition, delve into the concepts of atomic number, mass number, and isotopes, and examine how these factors influence sodium's reactivity and applications.
Understanding Atomic Structure: The Building Blocks of Matter
Before we delve into the specifics of sodium, let's establish a foundational understanding of atomic structure. An atom, the basic unit of a chemical element, is composed of three subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the element's atomic number and determines its chemical identity.
- Neutrons: Neutrally charged particles also residing in the nucleus. They contribute to the atom's mass but not its charge.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. The number of electrons usually equals the number of protons in a neutral atom.
The arrangement of these particles dictates an element's chemical properties and how it interacts with other elements. The interplay between protons and electrons, in particular, determines an atom's reactivity and its ability to form chemical bonds.
Sodium's Atomic Number and Protons
Sodium's atomic number is 11. This means that every sodium atom possesses 11 protons in its nucleus. This number is fundamental; it's what sets sodium apart from all other elements on the periodic table. No other element has 11 protons. This fixed number of protons is crucial in determining sodium's position on the periodic table, its electron configuration, and subsequently its chemical behavior.
The Significance of Atomic Number
The atomic number is more than just a number; it is the defining characteristic of an element. It dictates:
- Chemical Properties: The number of protons directly influences the number of electrons, which determines the atom's electron configuration and, thus, its chemical reactivity.
- Position on the Periodic Table: Elements are arranged on the periodic table in order of increasing atomic number, reflecting their fundamental chemical properties.
- Isotope Identification: While the number of protons remains constant for a given element, the number of neutrons can vary, leading to different isotopes. However, the atomic number always remains the same, regardless of the isotope.
Sodium's Mass Number and Neutrons
The mass number of an atom is the total number of protons and neutrons in its nucleus. Unlike the atomic number, which is always constant for a given element, the mass number can vary. This variation arises from the existence of isotopes.
While the most common isotope of sodium, Sodium-23 (²³Na), has 11 protons, it also has 12 neutrons. This gives it a mass number of 23 (11 protons + 12 neutrons). The mass number is often written as a superscript to the left of the element's symbol.
Isotopes: Variations in Neutron Number
Isotopes are atoms of the same element (same number of protons) that have different numbers of neutrons. This means they have the same atomic number but different mass numbers. Sodium has several isotopes, but ²³Na is by far the most abundant, making up over 99.9% of naturally occurring sodium. Other isotopes, such as ²²Na, are radioactive and have shorter half-lives.
The presence of different isotopes influences the average atomic mass reported on the periodic table. The average atomic mass is a weighted average of the masses of all the isotopes of an element, considering their relative abundances.
Sodium's Electrons and Chemical Reactivity
A neutral sodium atom has the same number of electrons as protons—11 electrons. These electrons are distributed in energy levels or shells around the nucleus. Sodium's electron configuration is 1s²2s²2p⁶3s¹. This means it has two electrons in the first shell, eight in the second shell, and one electron in the third shell.
The lone electron in the outermost shell (valence electron) is crucial to sodium's chemical behavior. This single valence electron is relatively loosely held and easily lost. This tendency to lose an electron makes sodium highly reactive, readily forming a +1 cation (Na⁺) to achieve a stable electron configuration similar to neon (a noble gas). This process is often represented in chemical reactions and ionic bonding.
The Role of Electrons in Chemical Bonding
The electrons, particularly the valence electrons, play a pivotal role in chemical bonding. Sodium's tendency to lose its valence electron to attain a stable octet (eight electrons in its outermost shell) is a driving force behind its reactivity. This is exemplified in its reactions with non-metals like chlorine, where sodium readily donates its valence electron to chlorine, forming an ionic bond resulting in sodium chloride (NaCl), common table salt.
Applications of Sodium and its Compounds
Sodium's unique properties, stemming from its atomic structure, make it crucial in numerous applications:
- Table Salt (NaCl): Sodium chloride is the most common sodium compound, used extensively in food preservation, seasoning, and various industrial processes.
- Sodium Hydroxide (NaOH): Also known as lye or caustic soda, it's used in soap making, paper production, and drain cleaning.
- Sodium Bicarbonate (NaHCO₃): Baking soda, used in baking as a leavening agent, in antacids, and in fire extinguishers.
- Sodium Lamps: These lamps emit a characteristic yellow light due to the excitation of sodium atoms, commonly used in street lighting.
- Biological Roles: Sodium ions (Na⁺) are essential for nerve impulse transmission, muscle contraction, and maintaining fluid balance in living organisms.
Summary: The Importance of Atomic Structure in Understanding Sodium
The number of protons, neutrons, and electrons in sodium is not just a set of numbers; it's the key to understanding its chemical properties and behavior. The 11 protons define it as sodium, the 12 neutrons in its most abundant isotope contribute to its mass, and the 11 electrons determine its reactivity and its ability to form chemical bonds, leading to its wide range of applications.
By grasping the fundamental principles of atomic structure, we gain insights into the macroscopic properties of sodium and its diverse roles in various fields, from everyday life to advanced technologies and biological systems. The seemingly simple composition of sodium—11 protons, 11 electrons, and usually 12 neutrons—underpins a remarkable array of applications and biological functions. The understanding of this atomic structure is essential not just for students of chemistry but for anyone curious about the world around us.
Latest Posts
Latest Posts
-
How To Make A Ratio Into A Percent
Mar 18, 2025
-
The Si Unit For Work Is The
Mar 18, 2025
-
How Many Valence Electrons Are In Be
Mar 18, 2025
-
Change State From Gas To Liquid
Mar 18, 2025
-
What Is The Force That Opposes Motion
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Many Protons Neutrons And Electrons In Sodium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
