Change State From Gas To Liquid
Juapaving
Mar 18, 2025 · 6 min read
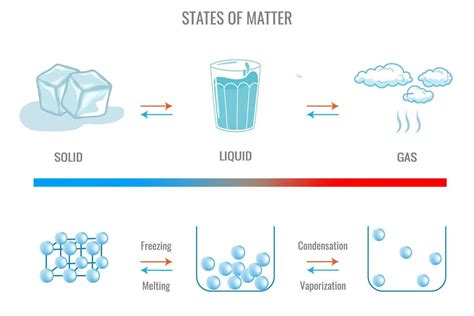
Table of Contents
The Fascinating Transformation: Understanding the Change of State from Gas to Liquid
The world around us is a constant dance of matter transitioning between its different states – solid, liquid, and gas. While we often observe water freezing and melting, the transformation from a gas to a liquid, a process known as condensation, is equally fascinating and crucial to numerous natural phenomena and industrial processes. This comprehensive guide will delve deep into the mechanics of condensation, exploring its underlying principles, influencing factors, and real-world applications.
Understanding the Gaseous State
Before we dive into the transition, let's refresh our understanding of gases. Gases are characterized by their lack of fixed shape or volume. Their particles are widely dispersed and possess high kinetic energy, constantly moving and colliding with each other and the walls of their container. This constant movement generates pressure. The behavior of ideal gases can be accurately predicted using the Ideal Gas Law (PV = nRT), which relates pressure (P), volume (V), number of moles (n), temperature (T), and the ideal gas constant (R). However, real gases deviate from this ideal behavior at high pressures and low temperatures.
Key Properties of Gases:
- High Kinetic Energy: Particles move rapidly and randomly.
- Weak Intermolecular Forces: Forces of attraction between gas particles are minimal.
- Compressibility: Gases can be easily compressed into smaller volumes.
- Expansion: Gases expand to fill the available volume.
- Diffusion: Gases readily mix with each other.
The Process of Condensation: From Gas to Liquid
Condensation is the phase transition where a substance in its gaseous state changes into its liquid state. This transformation occurs when the gas molecules lose sufficient kinetic energy to overcome the intermolecular attractive forces. As the gas cools, the particles slow down, and the intermolecular forces become significant enough to pull the particles closer together. This clustering of particles forms the liquid phase.
The Role of Intermolecular Forces:
The strength of intermolecular forces plays a crucial role in the condensation process. Stronger intermolecular forces, such as hydrogen bonding in water, lead to higher condensation temperatures. Weaker forces, such as van der Waals forces in noble gases, result in lower condensation temperatures. Understanding these forces is essential to predicting and controlling the condensation process.
Factors Affecting Condensation:
Several factors influence the rate and occurrence of condensation:
-
Temperature: Lowering the temperature is the primary driver of condensation. As temperature decreases, the kinetic energy of gas particles reduces, allowing intermolecular forces to dominate and draw the particles together.
-
Pressure: Increasing pressure forces gas molecules closer together, increasing the frequency of collisions and promoting condensation. Higher pressure enhances the likelihood of intermolecular interactions, leading to liquefaction.
-
Surface Area: Condensation preferentially occurs on surfaces. Larger surface areas provide more sites for gas molecules to adhere and initiate condensation. This is why dew forms on leaves and grass.
-
Presence of Condensation Nuclei: These are tiny particles, such as dust or ions, that provide surfaces for gas molecules to condense upon. They act as nucleation sites, accelerating the condensation process. Without them, condensation can be significantly slower, a phenomenon seen in cloud formation.
Understanding the Condensation Point (or Dew Point):
The condensation point, also known as the dew point, is the temperature at which a gas begins to condense into a liquid at a constant pressure. It represents the temperature at which the vapor pressure of the gas equals the partial pressure of the gas in the surrounding atmosphere. The dew point is a crucial parameter in meteorology, as it indicates the likelihood of fog or dew formation. A high dew point signifies high atmospheric moisture content, increasing the probability of condensation.
Real-World Examples of Condensation:
Condensation is a ubiquitous process observed in numerous natural and industrial settings:
Natural Phenomena:
-
Dew Formation: At night, the ground cools, lowering the temperature of the air near the surface. This leads to the condensation of atmospheric water vapor, forming dew on plants and other surfaces.
-
Fog and Cloud Formation: As warm, moist air rises and cools, it reaches its dew point, resulting in the condensation of water vapor into tiny water droplets or ice crystals, forming fog or clouds.
-
Rain and Snow: Clouds are composed of countless water droplets or ice crystals. When these droplets or crystals grow large enough to overcome updrafts, they fall as rain or snow.
-
Breath on a Cold Day: The warm, moist air expelled from our lungs cools rapidly upon contact with colder air, causing the water vapor to condense and form visible clouds of water droplets.
Industrial Applications:
-
Desalination: Condensation is crucial in desalination plants, where seawater is evaporated, and the resulting water vapor is then condensed to produce fresh water.
-
Liquefaction of Gases: Many gases, like natural gas and liquefied petroleum gas (LPG), are liquefied through condensation for easier storage and transport.
-
Refrigeration and Air Conditioning: Refrigerants in refrigerators and air conditioners undergo a cycle of evaporation and condensation to transfer heat and cool the environment.
-
Chemical Processes: Condensation is extensively used in many chemical processes to separate and purify substances.
The Reverse Process: Evaporation
It is important to understand that condensation is a reversible process. The opposite of condensation is evaporation (or vaporization), where a liquid transforms into a gas. Evaporation is driven by the kinetic energy of the liquid molecules. Molecules with sufficient kinetic energy can overcome the intermolecular forces and escape into the gaseous phase. Factors influencing evaporation include temperature, pressure, surface area, and the presence of air currents.
Applications of Understanding Condensation:
A thorough understanding of condensation has several significant applications across various disciplines:
-
Weather Forecasting: Accurate prediction of dew point and other condensation-related parameters is vital for weather forecasting.
-
Climate Modeling: Condensation processes significantly impact climate models, especially in predicting cloud formation and precipitation patterns.
-
Material Science: Understanding condensation is essential in designing materials that resist condensation or promote it, depending on the application. For instance, anti-fog coatings exploit the principles of condensation to prevent fog formation on surfaces.
-
Environmental Engineering: Condensation is crucial in designing efficient systems for air purification and humidity control.
Conclusion: The Importance of Condensation
Condensation is a fundamental phase transition with far-reaching implications. From the formation of dew and clouds to industrial processes like desalination and refrigeration, this process plays a vital role in shaping our environment and supporting numerous technological advancements. Understanding the underlying principles and factors influencing condensation is crucial for advancing various fields of science and engineering. Further research and development in this area will undoubtedly lead to innovative solutions in tackling global challenges related to water scarcity, climate change, and energy efficiency. The simple yet profound transformation from gas to liquid holds the key to unlocking a wealth of possibilities.
Latest Posts
Latest Posts
-
Is Salt A Compound Mixture Or Element
Mar 18, 2025
-
What Is The Lcm Of 24 And 14
Mar 18, 2025
-
Highest Common Factor Of 2 And 8
Mar 18, 2025
-
A Sentence That Shows Strong Or Sudden Feeling
Mar 18, 2025
-
Is Used To Split Things Apart
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Change State From Gas To Liquid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
