The Si Unit For Work Is The
Juapaving
Mar 18, 2025 · 6 min read
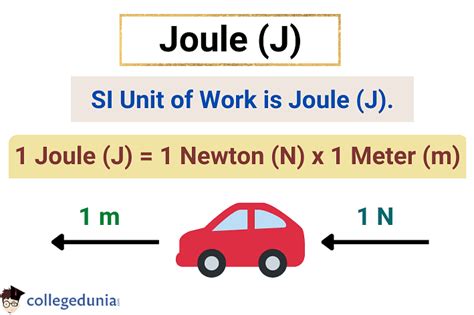
Table of Contents
The SI Unit for Work is the Joule: A Deep Dive into Energy and its Measurement
The fundamental concept of work in physics isn't just about toiling away at a desk. It's a precise measurement of energy transfer that occurs when a force causes an object to move a certain distance. Understanding work is crucial to comprehending numerous physical phenomena, from the simple act of lifting a weight to the complex workings of a power plant. And the cornerstone of this understanding lies in its unit of measurement: the Joule.
What is Work in Physics?
Before diving into the Joule, let's clarify the definition of work in a physics context. It's not simply any activity that expends effort. In physics, work is defined as the product of the force applied to an object and the distance the object moves in the direction of the force. This crucial "in the direction of the force" clause highlights a key distinction. If you push against a wall, you might exert considerable effort, but you're not doing any work on the wall because it's not moving.
Mathematically, work (W) is expressed as:
W = Fd cos θ
Where:
- W represents work, measured in Joules (J).
- F represents the force applied, measured in Newtons (N).
- d represents the displacement (distance moved) in meters (m).
- θ (theta) represents the angle between the force vector and the displacement vector.
This formula reveals a key aspect: only the component of the force acting in the direction of motion contributes to the work done. If the force is applied at an angle to the direction of motion, only the cosine component of the force is considered. If the force is perpendicular to the motion (θ = 90°), then cos θ = 0, and no work is done. For example, carrying a heavy box across a room at a constant height involves no vertical work, despite the considerable effort. The upward force you exert cancels out gravity, maintaining a constant height, and there is no net vertical displacement.
The Joule: A Comprehensive Look at the SI Unit of Work
The Joule (J), named after the 19th-century physicist James Prescott Joule, is the International System of Units (SI) unit for work, energy, and heat. One Joule is defined as the amount of work done when a force of one Newton is applied over a displacement of one meter in the direction of the force. Therefore:
1 Joule = 1 Newton-meter (N⋅m)
The Joule's versatility extends beyond simply measuring work. Because energy and work are fundamentally interchangeable (the work-energy theorem), the Joule is also the standard unit for measuring various forms of energy, including:
- Kinetic Energy: The energy an object possesses due to its motion. A moving car, a thrown ball, and even the Earth orbiting the sun all possess kinetic energy, measurable in Joules.
- Potential Energy: The energy an object possesses due to its position or configuration. A book on a shelf has gravitational potential energy, ready to be converted into kinetic energy if it falls. A stretched spring possesses elastic potential energy.
- Thermal Energy: The internal energy of a system related to the temperature and motion of its molecules. Heating an object increases its thermal energy, measured in Joules.
- Electrical Energy: The energy carried by moving electric charges. The energy delivered by a battery or generated by a power plant is measured in Joules.
Understanding the Significance of the Joule in Various Applications
The Joule's widespread applicability is a testament to its fundamental importance in physics and engineering. Let's explore some key applications:
1. Mechanics and Engineering
In mechanics, the Joule is crucial for analyzing the work done by machines, calculating the energy required to lift or move objects, and designing systems that efficiently transfer energy. Understanding work and energy allows engineers to optimize designs for maximum efficiency and minimize energy waste. For instance, calculating the work done in lifting a construction material using a crane involves applying the work formula directly.
2. Thermodynamics and Heat Transfer
In thermodynamics, the Joule is used to quantify heat transfer. The first law of thermodynamics states that the change in internal energy of a system is equal to the heat added to the system minus the work done by the system. This principle, expressed in Joules, is fundamental to understanding heat engines, refrigerators, and other thermodynamic systems. Calculating the heat required to raise the temperature of a material uses the concept of specific heat capacity and is ultimately expressed in Joules.
3. Electricity and Electronics
In electrical engineering, the Joule is used to measure electrical energy. Power (measured in Watts) is the rate of energy transfer, and 1 Watt is equivalent to 1 Joule per second (J/s). This means that a 100-watt lightbulb consumes 100 Joules of energy every second. The Joule is therefore central to understanding electricity bills and energy consumption.
4. Nuclear Physics
Even at the atomic level, the Joule finds its application. Nuclear reactions involve immense energy changes, often expressed in Joules, even if dealing with individual atoms. Understanding these energy changes is crucial for nuclear power generation and other nuclear applications.
Joule and Other Energy Units: Conversions and Practical Considerations
While the Joule is the SI unit, other energy units are commonly used, especially in specific contexts:
- Kilojoule (kJ): 1 kJ = 1000 J. Often used for larger energy quantities.
- Megajoule (MJ): 1 MJ = 1,000,000 J. Used for extremely large energy values.
- Calorie (cal): An older unit, primarily used in nutritional contexts. 1 cal ≈ 4.184 J.
- Kilocalorie (kcal) or Calorie (Cal): Often used in nutrition; 1 kcal = 1000 cal ≈ 4184 J. The "Calorie" on food labels is actually a kilocalorie.
- Kilowatt-hour (kWh): A unit of energy commonly used in electricity billing. 1 kWh = 3,600,000 J.
The ability to convert between these units is essential in practical applications. Understanding the relationships allows seamless transitions between different measurement systems and avoids confusion.
The Work-Energy Theorem: A Cornerstone of Classical Mechanics
The work-energy theorem is a crucial principle linking work and kinetic energy. It states that the net work done on an object is equal to the change in its kinetic energy. Mathematically:
W_net = ΔKE = ½mv² - ½mu²
where:
- W_net is the net work done on the object.
- ΔKE is the change in kinetic energy.
- m is the mass of the object.
- v is the final velocity of the object.
- u is the initial velocity of the object.
This theorem underscores the fundamental relationship between work and energy. The work done on an object directly affects its kinetic energy, highlighting the interchangeability between work and energy and reinforcing the significance of the Joule as the universal unit for both.
Conclusion: The Joule's Enduring Importance
The Joule, as the SI unit for work and energy, is far more than just a unit of measurement. It’s a fundamental concept that underpins our understanding of the physical world. From the simple act of lifting an object to the complex operations of power plants and the intricate processes within atoms, the Joule provides a consistent and universal framework for quantifying and analyzing energy transfer. Its significance is undeniable and enduring in various fields of science and engineering, making it a cornerstone of modern physics. The comprehensive understanding of the Joule and its applications is essential for anyone seeking a deep understanding of energy and its diverse manifestations.
Latest Posts
Latest Posts
-
5 Letter Words That Start With Ad
Mar 18, 2025
-
Words That End With An S
Mar 18, 2025
-
Five Letter Words Starting With S A L
Mar 18, 2025
-
Volume Is The Amount Of Space A
Mar 18, 2025
-
Which Of These Is A Chemical Change
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about The Si Unit For Work Is The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
