How Many Energy Levels Does Potassium Have
Juapaving
Mar 26, 2025 · 6 min read
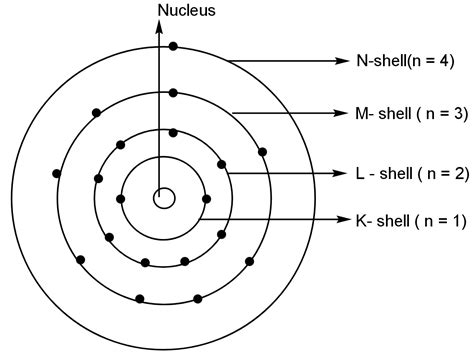
Table of Contents
How Many Energy Levels Does Potassium Have? Delving into Electron Configuration and Atomic Structure
Potassium, a vital element for human health and a common component in fertilizers, holds a fascinating place in the periodic table. Understanding its electronic structure, specifically the number of energy levels its electrons occupy, provides key insights into its chemical reactivity and properties. This article will delve deep into the electronic configuration of potassium, explaining its energy levels and how this influences its behavior.
Understanding Electron Shells and Energy Levels
Before we pinpoint the number of energy levels in potassium, let's establish a foundational understanding of electron shells and energy levels within an atom. An atom's electrons don't exist randomly; they occupy specific regions of space called electron shells or energy levels. These shells are arranged concentrically around the atom's nucleus, with each shell capable of holding a specific maximum number of electrons.
The shells are designated by integers (n=1, n=2, n=3, and so on), with n=1 representing the shell closest to the nucleus (the lowest energy level). As the value of 'n' increases, the energy level increases, and the electrons are further from the nucleus. The further an electron is from the nucleus, the higher its potential energy.
The maximum number of electrons a shell can hold is determined by the formula 2n², where 'n' is the shell number. Thus:
- Shell 1 (n=1): Holds a maximum of 2 electrons (2 x 1²)
- Shell 2 (n=2): Holds a maximum of 8 electrons (2 x 2²)
- Shell 3 (n=3): Holds a maximum of 18 electrons (2 x 3²)
- Shell 4 (n=4): Holds a maximum of 32 electrons (2 x 4²)
- And so on...
Potassium's Atomic Structure and Electron Configuration
Potassium (K) has an atomic number of 19, meaning it has 19 protons in its nucleus and 19 electrons orbiting the nucleus in a neutral atom. To determine the number of energy levels, we need to understand potassium's electron configuration, which describes how these 19 electrons are distributed among the different energy levels.
The electrons fill the energy levels in a specific order, starting with the lowest energy level (closest to the nucleus) and proceeding to higher energy levels. This filling follows the Aufbau principle, which states that electrons fill orbitals in order of increasing energy. The filling also adheres to Hund's rule and the Pauli exclusion principle, both governing electron behavior within orbitals.
Potassium's electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹. Let's break this down:
- 1s²: Two electrons in the first energy level (n=1) – the 1s subshell.
- 2s²: Two electrons in the second energy level (n=2) – the 2s subshell.
- 2p⁶: Six electrons in the second energy level (n=2) – the 2p subshell. The p subshell can hold up to six electrons.
- 3s²: Two electrons in the third energy level (n=3) – the 3s subshell.
- 3p⁶: Six electrons in the third energy level (n=3) – the 3p subshell.
- 4s¹: One electron in the fourth energy level (n=4) – the 4s subshell.
Determining the Number of Energy Levels in Potassium
From the electron configuration, it's clear that potassium's electrons occupy four energy levels: n=1, n=2, n=3, and n=4. The highest occupied energy level is the fourth energy level (n=4), which contains a single electron in the 4s subshell. This outermost electron is crucial in determining potassium's chemical reactivity.
Significance of the Outermost Electron
The single electron in the 4s subshell is a valence electron. Valence electrons are the electrons in the outermost shell of an atom and are responsible for the atom's chemical bonding and reactivity. Because potassium has only one valence electron, it readily loses this electron to achieve a stable electron configuration, resembling that of the noble gas argon. This makes potassium highly reactive and readily forms +1 ions.
Relating Energy Levels to Chemical Properties
The number of energy levels and the arrangement of electrons within those levels directly influence an element's chemical properties. Potassium's four energy levels contribute to its characteristic properties:
- Low Ionization Energy: Due to its single valence electron being relatively far from the nucleus, it requires relatively little energy to remove this electron. This low ionization energy explains potassium's high reactivity.
- Electropositivity: Potassium readily loses its valence electron to become a positively charged ion (K⁺). This tendency to lose electrons and form positive ions makes it electropositive.
- Reactivity with Water: Potassium reacts violently with water, releasing hydrogen gas. This vigorous reaction is a direct consequence of its readiness to lose its valence electron.
- Formation of Ionic Compounds: Potassium readily forms ionic compounds with nonmetals, such as potassium chloride (KCl) and potassium iodide (KI). These compounds are formed through the electrostatic attraction between the positively charged potassium ion and negatively charged nonmetal ions.
Beyond the Basics: Subshells and Orbitals
While we've focused on the main energy levels, it's important to briefly mention subshells and orbitals for a more complete picture. Within each energy level, there are subshells (s, p, d, f) which have slightly different energy levels. These subshells are further divided into orbitals, which are regions of space where there's a high probability of finding an electron.
Each orbital can hold a maximum of two electrons with opposite spins (Pauli Exclusion Principle). The s subshell contains one orbital, the p subshell contains three orbitals, the d subshell contains five orbitals, and the f subshell contains seven orbitals. Understanding this level of detail provides a more nuanced understanding of electron behavior and chemical bonding.
Potassium in Biological Systems and Applications
Potassium's unique electronic structure and resulting chemical properties are critical to its biological roles and various applications:
-
Biological Importance: Potassium is an essential electrolyte in living organisms, playing a vital role in maintaining proper fluid balance, nerve impulse transmission, and muscle contraction. Its involvement in these processes underscores the importance of its readily available valence electron and its ability to form ionic bonds.
-
Fertilizers: Potassium is a crucial nutrient for plants, promoting healthy growth and increasing crop yields. It's a key component in many fertilizers, highlighting the importance of its chemical reactivity and ability to form ionic compounds that are readily absorbed by plants.
-
Industrial Applications: Potassium compounds have various industrial applications, including the production of soaps, glass, and other chemicals. The unique chemical properties stemming from its electronic configuration make it suitable for diverse industrial processes.
Conclusion: Potassium's Energy Levels and Their Impact
In summary, potassium has four energy levels occupied by its 19 electrons. This electronic configuration, particularly the presence of a single valence electron in the outermost energy level, is fundamental to potassium's characteristic properties and its crucial roles in biological systems and various industrial applications. Understanding the energy levels and electron configuration helps us appreciate the relationship between atomic structure and macroscopic properties, providing a deeper insight into the fascinating world of chemistry. The readily available valence electron, a direct consequence of its electronic structure, dictates its high reactivity, its role as an essential electrolyte, and its widespread use in agriculture and industry. This highlights the profound interconnectedness of atomic structure, chemical behavior, and real-world applications.
Latest Posts
Latest Posts
-
Moment Of Inertia Of A Rectangular Plate
Mar 29, 2025
-
2 Lines Cut By A Transversal
Mar 29, 2025
-
Chemical Reaction Examples In Everyday Life
Mar 29, 2025
-
Twin Primes From 1 To 100
Mar 29, 2025
-
Is Butter Melting A Physical Or Chemical Change
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about How Many Energy Levels Does Potassium Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
