Osmosis Low To High Or High To Low
Juapaving
Mar 30, 2025 · 6 min read
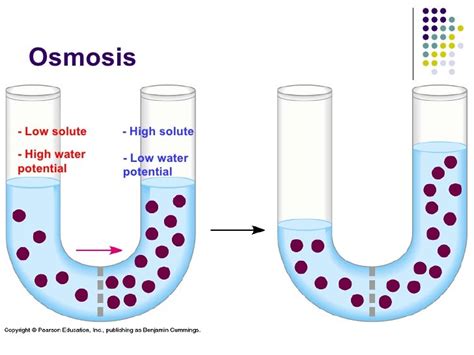
Table of Contents
Osmosis: The Movement of Water from Low to High Concentration? Or is it High to Low?
Understanding osmosis is crucial for grasping many biological processes. Often simplified, the concept is sometimes misrepresented, leading to confusion about the direction of water movement. This article will delve deep into the mechanics of osmosis, clarifying the seemingly contradictory statements you might encounter. We'll explore the underlying principles, dispel common misconceptions, and examine the role of osmosis in various biological systems.
Understanding Osmosis: The Basics
Osmosis is the passive transport of water across a selectively permeable membrane from a region of high water potential to a region of low water potential. It's vital to emphasize that this movement is driven by the difference in water potential, not the concentration of solutes directly.
Let's break that down:
-
Passive Transport: This means osmosis doesn't require energy input from the cell. It's a spontaneous process driven by the inherent properties of water molecules and their tendency to equalize concentration gradients.
-
Selectively Permeable Membrane: This is a crucial component. The membrane allows water to pass through but restricts the movement of larger solute molecules (like sugars or salts). This differential permeability is key to creating the water potential gradient that drives osmosis.
-
Water Potential: This is the measure of the free energy of water. Pure water has the highest water potential. Adding solutes lowers the water potential because the solute molecules bind to water molecules, reducing the number of free water molecules available to move. Think of it like this: the more solute you add, the "less free" the water becomes.
Therefore, water moves from an area where it's more "free" (high water potential, typically a solution with low solute concentration) to an area where it's less "free" (low water potential, typically a solution with high solute concentration). This is the key to understanding why water moves from a hypotonic solution to a hypertonic solution.
Misconceptions and Clarifications
The phrase "osmosis is the movement of water from high to low concentration" is often used and can be misleading. While it might seem intuitive, it's not entirely accurate. It's more precise to state that water moves from an area of high water potential to an area of low water potential. Here's why:
-
Focus on Water Potential, Not Solute Concentration: While solute concentration influences water potential, it's not the direct driving force. A solution can have a high concentration of solute and still have a higher water potential than another solution with a lower solute concentration if the other factors affecting water potential (like pressure) are different.
-
Water, Not Solutes, Moves: Osmosis is specifically about the movement of water. Solutes generally don't move across the membrane directly during simple osmosis. Their concentration gradient influences the water potential gradient, which then causes water movement.
-
The Role of Pressure: Water potential is affected by pressure. A high-pressure environment can increase water potential, even if the solute concentration is high. Conversely, a low-pressure environment can decrease water potential, even with low solute concentration.
Hypotonic, Hypertonic, and Isotonic Solutions: Understanding the Terminology
Understanding these terms is essential for comprehending osmosis in different scenarios:
-
Hypotonic Solution: A solution with a higher water potential than the cell's interior. This means the solution has a lower solute concentration than the cell. Water will move into the cell via osmosis, causing it to swell or even burst (lyse) if the cell wall is not strong enough.
-
Hypertonic Solution: A solution with a lower water potential than the cell's interior. This means the solution has a higher solute concentration than the cell. Water will move out of the cell via osmosis, causing it to shrink (crenate) or plasmolyze (in plant cells, where the cell membrane pulls away from the cell wall).
-
Isotonic Solution: A solution with the same water potential as the cell's interior. There is no net movement of water across the membrane; the cell remains stable.
Osmosis in Biological Systems: Examples and Implications
Osmosis plays a critical role in numerous biological processes, including:
1. Plant Cells and Turgor Pressure:
Plant cells rely heavily on osmosis to maintain their turgor pressure. When plant cells are in a hypotonic environment, water enters the cell, creating turgor pressure against the cell wall. This pressure provides structural support, keeping the plant upright. If the plant is in a hypertonic environment, water leaves the cells, leading to wilting (plasmolysis).
2. Water Uptake by Roots:
Plants absorb water from the soil through their roots via osmosis. The root cells maintain a lower water potential than the soil water, causing water to move into the roots and then up the plant.
3. Maintaining Cell Shape and Volume:
Osmosis helps maintain the proper shape and volume of cells. Cells are constantly adjusting their internal solute concentrations to balance water movement and prevent excessive swelling or shrinkage.
4. Kidney Function:
The kidneys use osmosis to regulate water balance in the body. The nephrons in the kidneys reabsorb water from the filtrate, preventing excessive water loss in urine.
5. Nutrient Absorption in the Intestines:
Osmosis facilitates the absorption of nutrients in the intestines. The concentration gradients created by the digestive process drive the movement of water and nutrients into the bloodstream.
Reverse Osmosis: A Practical Application
Reverse osmosis is a process that uses pressure to force water across a semi-permeable membrane against its natural osmotic gradient. This is used to purify water by removing impurities like salts and minerals. It requires energy input, unlike natural osmosis.
Factors Affecting Osmosis Rate
Several factors influence the rate of osmosis:
-
Concentration Gradient: A steeper concentration gradient (larger difference in water potential) leads to a faster rate of osmosis.
-
Temperature: Higher temperatures generally increase the rate of osmosis, as water molecules move more rapidly.
-
Surface Area of the Membrane: A larger surface area allows for faster water movement.
-
Membrane Permeability: The permeability of the membrane to water affects the rate of osmosis. More permeable membranes allow for faster osmosis.
Conclusion: A Clearer Picture of Osmosis
Osmosis is a fundamental biological process driven by the difference in water potential across a selectively permeable membrane. It's essential to emphasize that water movement occurs from a region of high water potential to a region of low water potential, not simply from high to low solute concentration. Understanding this distinction is key to comprehending the diverse roles osmosis plays in maintaining cellular function and overall organismal health. By grasping the underlying principles and related terminology, we can better appreciate the intricate mechanisms that govern life at a cellular level and beyond. This understanding extends to various practical applications, such as water purification technologies and medical treatments. Therefore, continue to explore and deepen your understanding of this crucial biological process.
Latest Posts
Latest Posts
-
How Many Feet Are In 40 Inches
Apr 01, 2025
-
Organisms That Make Their Own Food Is Called
Apr 01, 2025
-
How To Multiply By A Reciprocal
Apr 01, 2025
-
How Does Passive Transport Differ From Active Transport
Apr 01, 2025
-
Dilated By A Scale Factor Of 1 2
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Osmosis Low To High Or High To Low . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
