How To Find The Neutron Number
Juapaving
Mar 30, 2025 · 6 min read
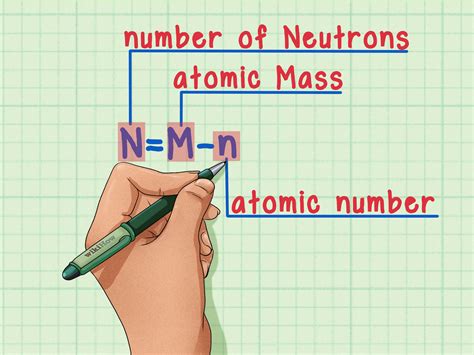
Table of Contents
How to Find the Neutron Number: A Comprehensive Guide
Determining the neutron number of an atom is a fundamental concept in chemistry and physics. Understanding this number is crucial for comprehending nuclear reactions, isotopic variations, and the properties of elements. This comprehensive guide will walk you through various methods of finding the neutron number, explaining the underlying principles and providing practical examples.
Understanding Atomic Structure and Notation
Before diving into the methods, let's solidify our understanding of atomic structure. An atom consists of a nucleus containing protons and neutrons, orbited by electrons.
- Protons: Positively charged particles; determine the element's atomic number (Z).
- Neutrons: Neutral particles; contribute to the atom's mass but not its charge.
- Electrons: Negatively charged particles; orbit the nucleus and participate in chemical reactions.
The atom's mass number (A) represents the total number of protons and neutrons in the nucleus. We typically represent an atom using the following notation: ^A_Z X, where:
- X is the element's chemical symbol (e.g., H for hydrogen, O for oxygen).
- Z is the atomic number (number of protons).
- A is the mass number (number of protons + neutrons).
The Primary Method: Using Mass Number and Atomic Number
The most straightforward method for determining the neutron number (N) involves using the mass number (A) and the atomic number (Z). The relationship is simple:
N = A - Z
This equation highlights that the neutron number is the difference between the total number of nucleons (protons and neutrons) and the number of protons.
Example 1: Carbon-12
Carbon-12 has an atomic number (Z) of 6 (meaning it has 6 protons) and a mass number (A) of 12. Therefore, its neutron number (N) is:
N = 12 - 6 = 6
Carbon-12 has 6 neutrons.
Example 2: Uranium-235
Uranium-235, a crucial isotope in nuclear reactors, has an atomic number (Z) of 92 and a mass number (A) of 235. Calculating its neutron number:
N = 235 - 92 = 143
Uranium-235 possesses 143 neutrons.
Understanding Isotopes and Their Neutron Numbers
Isotopes are atoms of the same element (same atomic number) but with different numbers of neutrons (and therefore different mass numbers). This variation in neutron number affects the atom's stability and properties.
Example 3: Hydrogen Isotopes
Hydrogen has three isotopes:
- Protium (¹H): Z = 1, A = 1, N = 0 (one proton, no neutrons)
- Deuterium (²H): Z = 1, A = 2, N = 1 (one proton, one neutron)
- Tritium (³H): Z = 1, A = 3, N = 2 (one proton, two neutrons)
These isotopes demonstrate how varying neutron numbers can exist within the same element. Notice how the neutron number directly impacts the mass number and influences the isotope's characteristics.
Advanced Techniques: Mass Spectrometry and Nuclear Reactions
While the A - Z method is commonly used, more advanced techniques are employed in specific research contexts.
1. Mass Spectrometry:
Mass spectrometry is a powerful analytical technique that measures the mass-to-charge ratio of ions. By analyzing the mass spectrum of a sample, scientists can determine the relative abundance of different isotopes and indirectly infer the neutron number of each isotope. The precise mass measurement allows for highly accurate determination of isotopic composition, which, coupled with the known atomic number, allows for precise calculation of the neutron number. Sophisticated mass spectrometers can differentiate between isotopes with minute mass differences, providing invaluable data for nuclear studies.
2. Nuclear Reactions:
Nuclear reactions, such as neutron capture or beta decay, change the number of neutrons in an atom's nucleus. By observing and analyzing these reactions, researchers can deduce the neutron number of the resulting isotopes. For instance, neutron capture increases the neutron number by one, while beta decay (converting a neutron to a proton) decreases the neutron number by one while increasing the proton number by one. Analyzing the products of these reactions, along with the initial reactants, allows scientists to determine the neutron numbers involved.
Importance of Neutron Number
The neutron number plays a pivotal role in various aspects of nuclear science and chemistry:
-
Nuclear Stability: The ratio of neutrons to protons significantly impacts an atom's stability. Too many or too few neutrons relative to protons can lead to radioactive decay. The "stable isotope" band on the chart of nuclides represents the optimal neutron-to-proton ratios for stable nuclei.
-
Nuclear Reactions: Neutron number is crucial in understanding nuclear reactions, including fission and fusion. The specific number of neutrons significantly influences the likelihood and characteristics of these processes. For example, the ability of uranium-235 to undergo fission is directly linked to its neutron number.
-
Isotopic Properties: Isotopes of the same element exhibit similar chemical properties due to identical electron configurations, but their physical properties can differ considerably due to differing neutron numbers and therefore masses. These differences in mass affect factors such as density, melting point, and boiling point.
-
Nuclear Medicine: Specific isotopes with particular neutron numbers are used in medical applications, such as diagnostic imaging and radiation therapy. Understanding the neutron number is crucial for selecting suitable isotopes for these purposes.
Potential Challenges and Considerations
While determining the neutron number is relatively straightforward using the A - Z method, some considerations are important:
-
Accuracy of Mass Number: The accuracy of the neutron number calculation depends on the accuracy of the mass number (A) measurement. In some cases, the mass number might be an average representing a mixture of isotopes, leading to slight inaccuracies in the calculated neutron number for individual isotopes. Advanced techniques, such as mass spectrometry, are employed for precise mass number determination.
-
Radioactive Decay: Radioactive isotopes undergo transformations that change their neutron and proton numbers. For unstable isotopes, the neutron number may not remain constant over time. In such cases, the neutron number refers to the isotope’s initial state, prior to any decay.
-
High Atomic Numbers: For elements with high atomic numbers, the neutron-to-proton ratio required for stability deviates significantly from 1:1. Accurate determination of neutron numbers becomes increasingly important for understanding the stability and behaviour of heavy nuclei.
Conclusion
Determining the neutron number is a fundamental skill in atomic physics and chemistry. The most common approach, subtracting the atomic number from the mass number, offers a straightforward way to find it. However, advanced techniques such as mass spectrometry and analysis of nuclear reactions provide more precise measurements and detailed insights. Understanding the neutron number is crucial for comprehending nuclear stability, isotopic properties, nuclear reactions, and various applications, especially in nuclear medicine and energy. This guide provides a thorough understanding of the concept and various methods for obtaining the neutron number, empowering students and researchers alike to approach this topic confidently. Remember to always consider potential challenges and utilize appropriate techniques for accurate and reliable results.
Latest Posts
Latest Posts
-
A Transverse Pulse Generated At The Bottom
Apr 01, 2025
-
Nitric Acid And Sodium Hydroxide Balanced Equation
Apr 01, 2025
-
Lowest Common Denominator Of 4 And 9
Apr 01, 2025
-
Dna Is Found In What Two Organelles
Apr 01, 2025
-
What Are The Factor Pairs Of 54
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How To Find The Neutron Number . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
