How Many Electrons Can F Hold
Juapaving
Mar 18, 2025 · 6 min read
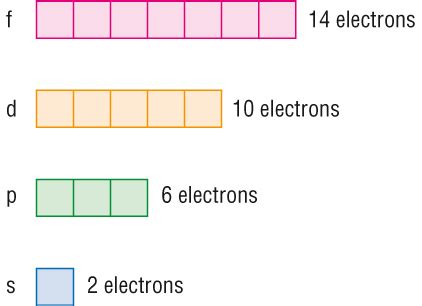
Table of Contents
How Many Electrons Can an f Subshell Hold? A Deep Dive into Atomic Structure
Understanding the electron configuration of atoms is fundamental to chemistry and physics. A key aspect of this understanding involves grasping the capacity of different subshells within an atom's electron cloud. This article delves deep into the 'f' subshell, exploring its structure, electron capacity, and the implications for the periodic table and the properties of elements. We'll uncover why the 'f' subshell can hold a specific number of electrons and how this impacts the behavior of the elements it populates.
The Quantum Mechanical Model and Subshells
Before we dive into the 'f' subshell, let's briefly review the quantum mechanical model of the atom. This model describes electrons not as orbiting the nucleus in neat paths, but rather as existing in regions of probability called orbitals. These orbitals are grouped into subshells, which are further grouped into electron shells.
Each subshell is designated by a letter: s, p, d, and f. The number of orbitals and, consequently, the maximum number of electrons each subshell can hold, is determined by the quantum numbers.
-
Principal Quantum Number (n): This number determines the energy level and size of the shell. It can be any positive integer (1, 2, 3,...).
-
Azimuthal Quantum Number (l): This number determines the shape of the subshell and its angular momentum. It can range from 0 to n-1. The values of l correspond to the subshells as follows: l=0 (s), l=1 (p), l=2 (d), l=3 (f).
-
Magnetic Quantum Number (ml): This number determines the orientation of the orbital in space. It can range from -l to +l, including 0.
-
Spin Quantum Number (ms): This number describes the intrinsic angular momentum of the electron, with values of +1/2 or -1/2 (spin up or spin down).
The 'f' Subshell: Orbitals and Electron Capacity
The 'f' subshell is characterized by an azimuthal quantum number (l) of 3. This means that it has 2l + 1 = 2(3) + 1 = 7 orbitals. Each orbital can hold a maximum of two electrons (due to the Pauli Exclusion Principle, which states that no two electrons in an atom can have the same set of four quantum numbers).
Therefore, the maximum number of electrons an 'f' subshell can hold is 14. This is a crucial number in understanding the structure and properties of the lanthanides and actinides, the two series of elements that fill the 'f' subshells.
Lanthanides and Actinides: The 'f'-block Elements
The elements in the periodic table that are characterized by the filling of the 'f' subshells are the lanthanides (also known as rare earth elements) and the actinides. These elements are located in the separate rows below the main body of the periodic table because including them in the main body would make the table excessively wide and unwieldy.
Lanthanides: These elements (atomic numbers 57-71) fill the 4f subshell. As the 4f subshell is gradually filled, the electrons are added to orbitals within this subshell. This gradual filling results in subtle differences in the properties of these elements, though they share many similarities due to their similar outer electron configurations.
Actinides: These elements (atomic numbers 89-103) fill the 5f subshell. Similar to the lanthanides, the actinides exhibit similarities in their properties due to the gradual filling of their 5f subshells. However, the actinides are generally more radioactive and less stable than the lanthanides.
Implications of the 14 Electron Capacity
The fact that the 'f' subshell can hold 14 electrons has significant implications:
-
Periodic Table Structure: The 14 elements in each of the lanthanide and actinide series directly result from the capacity of the 'f' subshells. The periodic table's arrangement reflects this filling order.
-
Electron Shielding: The 'f' electrons are relatively poor at shielding the outer electrons from the nuclear charge. This leads to a phenomenon called the lanthanide contraction, where the atomic radius decreases across the lanthanide series. A similar effect occurs across the actinide series, though less pronounced.
-
Chemical Properties: The relatively poor shielding of 'f' electrons influences the chemical properties of the lanthanides and actinides. They tend to exhibit similar chemical behavior, often forming similar compounds and exhibiting similar oxidation states. However, slight variations in electron configuration and shielding do lead to some differences in reactivity and other properties.
-
Magnetic Properties: Many lanthanides and actinides exhibit interesting magnetic properties due to their unpaired 'f' electrons. These unpaired electrons contribute to the overall magnetic moment of the atom. This is why some of these elements are used in specialized magnets.
-
Applications: The unique properties of lanthanides and actinides, stemming from their 'f' electron configurations, lead to diverse applications. Lanthanides are used in high-strength permanent magnets, catalysts, and lighting. Actinides, due to their radioactivity, are used in nuclear power and medical applications (though with caution due to their hazardous nature).
Beyond the Basics: Further Considerations
While the maximum electron capacity of an 'f' subshell is 14, it's important to remember that the actual number of electrons in an 'f' subshell will vary depending on the element and its atomic number. In many cases, the 'f' subshell is only partially filled. The energy levels of the 'f' orbitals are relatively close to other orbitals, leading to complex interactions and electron configurations that are not always straightforward.
The Role of Electron-Electron Repulsion
Electron-electron repulsion plays a significant role in determining the actual electron configuration of elements, particularly those with partially filled 'f' subshells. Electrons repel each other due to their like charges. This repulsion can influence which orbitals are filled first and can lead to exceptions to the expected filling order predicted by simple rules.
Relativistic Effects in Actinides
In the heavier actinide elements, relativistic effects become more significant. Relativistic effects arise from the very high speeds of electrons close to the nucleus. These effects influence the energy levels and sizes of the orbitals, particularly the 'f' orbitals. This can lead to deviations from the trends observed in the lanthanides.
Conclusion: Understanding the 'f' Subshell's Significance
The 'f' subshell, with its capacity to hold 14 electrons, is crucial for understanding the structure of the periodic table and the properties of the lanthanides and actinides. Its relatively poor shielding effect, combined with the complexities of electron-electron repulsion and relativistic effects in heavier elements, contributes to the unique and often fascinating properties of these elements. From their applications in high-tech magnets to their role in nuclear technology, the 14 electrons in the 'f' subshell significantly impact our world. Further research into the intricacies of electron configuration and the subtle effects within the 'f' block continues to reveal new insights and applications. Understanding the fundamental principles governing electron behavior is paramount to advancing our knowledge of the atom and its role in shaping the world around us.
Latest Posts
Latest Posts
-
Least Common Multiple Of 10 And 7
Mar 18, 2025
-
What Are The 3 Types Of Wires
Mar 18, 2025
-
What Is The Smallest Form Of Matter
Mar 18, 2025
-
Examples For Push And Pull Forces
Mar 18, 2025
-
Which Of The Following Is Secondary Pollutant
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Can F Hold . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
