How Many Electrons Are In Carbon's Valence Shell
Juapaving
Mar 24, 2025 · 5 min read
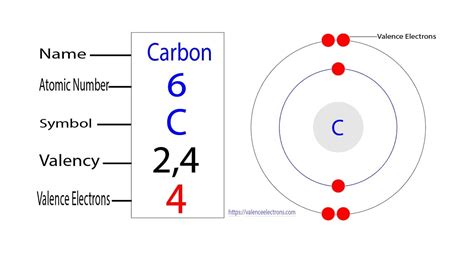
Table of Contents
How Many Electrons Are in Carbon's Valence Shell? Understanding Carbon's Bonding Behavior
Carbon, the backbone of organic chemistry and a cornerstone element in all known life forms, boasts a unique electronic structure that dictates its remarkable versatility in forming chemical bonds. Understanding the number of electrons in carbon's valence shell is crucial to comprehending its behavior and its ability to create the complex molecules that make up our world. This comprehensive guide will delve into the intricacies of carbon's electronic configuration, explain its valence electrons, and explore the implications for its bonding capabilities.
Understanding Atomic Structure and Electron Shells
Before we dive into carbon's valence shell, let's establish a fundamental understanding of atomic structure. Atoms are composed of three subatomic particles: protons, neutrons, and electrons. Protons and neutrons reside in the atom's nucleus, while electrons occupy specific energy levels or shells surrounding the nucleus. These shells are designated by principal quantum numbers (n = 1, 2, 3, etc.), with each shell having a maximum capacity for electrons.
The first shell (n=1) can hold a maximum of two electrons, the second shell (n=2) can hold up to eight, and the third shell (n=3) can hold up to 18. The distribution of electrons among these shells determines an atom's chemical properties and its reactivity. This distribution is depicted in the atom's electron configuration.
Carbon's Electron Configuration: Unveiling the Mystery
Carbon, with an atomic number of 6, possesses six protons and six electrons in its neutral state. These six electrons are distributed among energy levels according to the Aufbau principle and Hund's rule. The electron configuration of carbon is: 1s²2s²2p².
Let's break this down:
- 1s²: This signifies that the first shell (n=1) contains two electrons in the 's' subshell. The 's' subshell is a spherical orbital capable of holding a maximum of two electrons.
- 2s²: The second shell (n=2) contains two electrons in the 's' subshell.
- 2p²: The second shell also contains two electrons in the 'p' subshell. The 'p' subshell is composed of three dumbbell-shaped orbitals (px, py, pz), each capable of holding up to two electrons, giving a total capacity of six electrons.
Identifying Valence Electrons: The Key to Reactivity
Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the most loosely bound to the nucleus and are therefore most involved in chemical bonding. They determine an atom's reactivity and its ability to form chemical bonds with other atoms.
In carbon's case, its outermost shell is the second shell (n=2), which contains a total of four electrons (two in the 2s subshell and two in the 2p subshell). Therefore, carbon has four valence electrons.
The Significance of Four Valence Electrons: Carbon's Bonding Prowess
The presence of four valence electrons is the key to carbon's exceptional bonding versatility. Carbon atoms can achieve a stable electron configuration by either gaining, losing, or sharing electrons with other atoms. However, due to its intermediate electronegativity, neither gaining nor losing four electrons is energetically favorable for carbon.
Covalent Bonding: Carbon's Preferred Strategy
The most common way carbon achieves a stable octet (eight electrons in its outermost shell) is through covalent bonding. In covalent bonding, carbon shares its four valence electrons with other atoms, forming strong covalent bonds. This sharing allows each atom to effectively complete its outermost electron shell, attaining a stable configuration similar to that of a noble gas.
This ability to form four covalent bonds allows carbon to form a vast array of molecules, ranging from simple molecules like methane (CH₄) to complex macromolecules like proteins and DNA.
Hybridization: Expanding Bonding Possibilities
Carbon's bonding capabilities are further enhanced by a phenomenon called hybridization. Hybridization involves the mixing of atomic orbitals to form new hybrid orbitals with different shapes and energies. In carbon, the 2s and 2p orbitals hybridize to form various hybrid orbitals, such as sp, sp², and sp³, which allow carbon to form different geometries of molecules, impacting their properties.
- sp hybridization: Results in linear geometry (e.g., acetylene).
- sp² hybridization: Results in trigonal planar geometry (e.g., ethylene).
- sp³ hybridization: Results in tetrahedral geometry (e.g., methane).
Carbon's Role in Organic Chemistry and Life
The unique properties arising from its four valence electrons make carbon the central atom in organic chemistry. The immense variety of organic molecules – hydrocarbons, alcohols, aldehydes, ketones, carboxylic acids, amines, amides, and countless others – is a direct consequence of carbon's ability to form strong covalent bonds with itself and other elements.
This capability underlies the complexity of life. Carbon's capacity to form long chains, branched structures, and rings creates the diverse range of molecules essential for biological functions. Proteins, carbohydrates, lipids, and nucleic acids – the fundamental building blocks of life – are all based on carbon backbones.
Beyond Organic Chemistry: Carbon's Wider Applications
Carbon's versatility extends far beyond organic chemistry. It is a key component in numerous materials with diverse applications:
- Diamonds: The strong covalent bonds in diamond create a highly rigid and hard material.
- Graphite: Graphite's layered structure makes it an excellent lubricant and conductor of electricity.
- Fullerenes: These cage-like structures exhibit unique electronic and mechanical properties.
- Carbon nanotubes: These cylindrical structures are exceptionally strong and have applications in electronics and materials science.
- Carbon fiber: Used in high-strength, lightweight composites for aerospace and automotive applications.
Conclusion: The Unsurpassed Importance of Carbon's Four Valence Electrons
The number of electrons in carbon's valence shell – four – is a defining characteristic that dictates its exceptional chemical behavior and versatility. This seemingly simple fact underpins the vast complexity of organic chemistry and the very existence of life as we know it. Understanding this fundamental aspect of carbon's electronic structure provides the foundation for comprehending the diverse properties and applications of this remarkable element. From the intricate molecules of living organisms to the groundbreaking materials of modern technology, carbon's four valence electrons continue to shape our world in profound ways. Further exploration into the field of chemistry will unveil even more fascinating intricacies of carbon bonding and its ubiquitous presence in our world.
Latest Posts
Latest Posts
-
Is 53 A Prime Number Or A Composite Number
Mar 28, 2025
-
What Is The Least Common Multiple Of 24 And 15
Mar 28, 2025
-
What Percent Is 2 3 Of A Circle
Mar 28, 2025
-
Ray Diagrams Of A Concave Mirror
Mar 28, 2025
-
Are The Diagonals Of An Isosceles Trapezoid Congruent
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Are In Carbon's Valence Shell . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
