How Many Carbon Atoms Are In Propane
Juapaving
Mar 28, 2025 · 5 min read
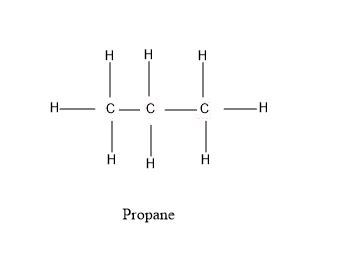
Table of Contents
How Many Carbon Atoms are in Propane? A Deep Dive into Alkane Chemistry
Propane. The name conjures images of gas grills sizzling on summer evenings, RV adventures, and perhaps even the slightly pungent aroma of a leaking gas line. But beyond its practical applications, propane holds a fascinating place in the world of chemistry, particularly in understanding the fundamental building blocks of organic molecules: carbon atoms. So, how many carbon atoms are in propane? The simple answer is three. But let's delve much deeper than that, exploring the molecular structure, nomenclature, and broader implications of this seemingly simple compound.
Understanding the Basics: Alkanes and their Structure
Before we dissect propane, it's crucial to understand its chemical family: the alkanes. Alkanes are saturated hydrocarbons – meaning they consist solely of carbon and hydrogen atoms, with each carbon atom forming four single bonds. This saturated nature dictates a specific, predictable structure, crucial for understanding the number of carbons in any given alkane.
The simplest alkane is methane (CH₄), with one carbon atom. Then comes ethane (C₂H₆) with two, followed by propane (C₃H₈), and so on. Each successive alkane in the series adds another carbon atom and two hydrogen atoms to the chain. This predictable pattern forms the basis for the alkane naming system.
The Linear Structure of Propane
Propane's chemical formula, C₃H₈, tells us it contains three carbon atoms and eight hydrogen atoms. Crucially, these atoms aren't arranged randomly. In propane, the three carbon atoms are arranged in a linear chain, with each carbon atom bonded to its neighbors. The remaining bonds of each carbon atom are saturated with hydrogen atoms.
This linear structure is vital. While isomers of propane exist (molecules with the same chemical formula but different structural arrangements), they're not considered in the basic understanding of "how many carbon atoms are in propane?". The standard, most common form of propane is the linear three-carbon chain.
Naming and Identifying Alkanes: The IUPAC System
The International Union of Pure and Applied Chemistry (IUPAC) established a systematic nomenclature for organic compounds, ensuring consistent naming across the scientific community. Understanding this system helps us confidently identify the number of carbon atoms in any alkane.
For alkanes, the naming follows a straightforward pattern:
- Meth- (1 carbon)
- Eth- (2 carbons)
- Prop- (3 carbons)
- But- (4 carbons)
- Pent- (5 carbons)
- Hex- (6 carbons)
- Hept- (7 carbons)
- Oct- (8 carbons)
- Non- (9 carbons)
- Dec- (10 carbons)
and so on. Adding the suffix "-ane" indicates that the compound is an alkane. Therefore, "propane" clearly signifies an alkane with three carbon atoms.
Beyond the Count: Exploring the Properties of Propane
Knowing propane contains three carbon atoms is just the starting point. This seemingly simple fact underpins many of propane's significant properties:
Physical Properties:
-
Gas at Room Temperature: Propane's relatively small size and weak intermolecular forces lead to its gaseous state under standard conditions. This is a key factor in its applications as a fuel.
-
Liquefaction under Pressure: Increasing pressure allows propane to be liquefied, making it easier to store and transport in cylinders. This property is fundamental to its widespread use.
-
Flammability: Propane readily combusts in the presence of oxygen, releasing significant energy in the form of heat and light. This exothermic reaction is the basis of its use as a fuel source for cooking, heating, and vehicles.
Chemical Properties:
-
Combustion: As mentioned, propane's complete combustion produces carbon dioxide and water, releasing energy. Incomplete combustion, due to insufficient oxygen, can result in the formation of carbon monoxide, a highly toxic gas. Understanding this is crucial for safe propane handling.
-
Halogenation: Propane can undergo halogenation reactions, where hydrogen atoms are replaced by halogen atoms (fluorine, chlorine, bromine, or iodine). This process leads to the formation of various haloalkanes, some of which have industrial applications.
-
Isomerization: While propane itself doesn't have isomers in the simplest linear form, larger alkanes can exhibit isomerism, significantly impacting their properties. Studying isomerism helps us understand the vast diversity within organic chemistry.
Propane's Applications: From Grills to Industry
The presence of three carbon atoms in propane is not merely an academic detail; it's a fundamental characteristic that directly influences its numerous applications:
-
Fuel for Grills and Stoves: Propane's clean burning and readily available liquefied form make it an ideal fuel for outdoor cooking and indoor heating appliances.
-
Automotive Fuel: Propane autogas is gaining popularity as a cleaner-burning alternative to gasoline, reducing emissions and contributing to a greener environment.
-
Industrial Applications: Propane serves as a feedstock for the petrochemical industry, used in the production of plastics, solvents, and other chemicals.
-
Refrigeration and Air Conditioning: Propane is used as a refrigerant in some applications, taking advantage of its thermodynamic properties.
Conclusion: The Significance of Three Carbon Atoms
The seemingly simple question – how many carbon atoms are in propane? – opens a door to a much broader understanding of organic chemistry. The answer, three, is not just a number; it's the foundation upon which propane's unique properties and vast applications are built. From the structure and nomenclature dictated by its three-carbon chain to its chemical reactivity and widespread industrial use, the number of carbon atoms in propane directly shapes its significance in the world around us. Understanding this simple detail allows us to appreciate the complex interplay between molecular structure, chemical properties, and practical applications, highlighting the fundamental importance of even the seemingly simplest organic molecules. Further exploration into the world of alkanes and organic chemistry reveals an even richer tapestry of connections and possibilities, all underpinned by the basic building blocks – the carbon atoms.
Latest Posts
Latest Posts
-
What Percent Is Equal To 3 8
Mar 30, 2025
-
Compare And Contrast Spermatogenesis And Oogenesis In Human Cells
Mar 30, 2025
-
What Is The Least Common Multiple Of 12 And 24
Mar 30, 2025
-
Find Area Under The Curve Calculator
Mar 30, 2025
-
Friction Always Works Blank The Direction Of Velocity
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about How Many Carbon Atoms Are In Propane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
