Which Law Represents A Balanced Chemical Equation
Juapaving
Mar 31, 2025 · 5 min read
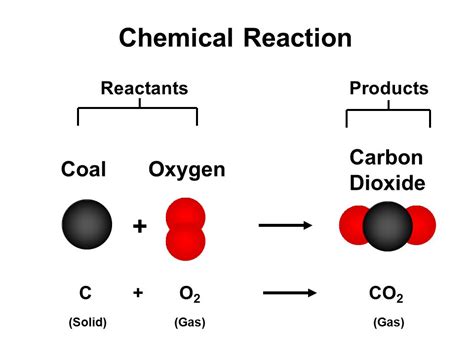
Table of Contents
Which Law Represents a Balanced Chemical Equation?
The Law of Conservation of Mass perfectly represents a balanced chemical equation. This fundamental principle in chemistry dictates that matter cannot be created or destroyed in a chemical reaction; it only changes form. A balanced chemical equation visually demonstrates this law by ensuring that the number of atoms of each element remains the same on both sides of the equation – the reactants (starting materials) and the products (resulting substances). Understanding this connection is crucial for mastering stoichiometry, predicting reaction outcomes, and comprehending the intricate dance of atoms during chemical transformations.
Understanding Chemical Equations
Before delving into the law's representation, let's solidify our understanding of chemical equations. A chemical equation is a symbolic representation of a chemical reaction. It uses chemical formulas to depict the reactants and products involved. For instance:
Reactants → Products
The arrow signifies the transformation from reactants to products. For example, the combustion of methane can be represented as:
CH₄ + O₂ → CO₂ + H₂O
This equation, however, is unbalanced. It doesn't reflect the Law of Conservation of Mass. To accurately represent the reaction, we need to balance it.
Balancing Chemical Equations: The Key to Representing the Law of Conservation of Mass
Balancing a chemical equation involves adjusting the coefficients (the numbers placed before the chemical formulas) to ensure that the number of atoms of each element is equal on both sides. Let's balance the methane combustion equation:
Unbalanced: CH₄ + O₂ → CO₂ + H₂O
Balanced: CH₄ + 2O₂ → CO₂ + 2H₂O
Notice how we added coefficients. Now:
- Carbon (C): 1 carbon atom on each side.
- Hydrogen (H): 4 hydrogen atoms on each side.
- Oxygen (O): 4 oxygen atoms on each side.
The balanced equation now adheres to the Law of Conservation of Mass. The total mass of the reactants (methane and oxygen) equals the total mass of the products (carbon dioxide and water). This is the essence of how a balanced chemical equation visually demonstrates this crucial law.
The Law of Conservation of Mass: A Cornerstone of Chemistry
The Law of Conservation of Mass, formulated by Antoine Lavoisier in the late 18th century, is a cornerstone of chemistry. It's not just a theoretical concept; it's an experimentally verifiable principle. Countless experiments have shown that in a closed system (where no matter enters or leaves), the total mass remains constant throughout a chemical reaction. This observation led to the understanding that chemical reactions involve the rearrangement of atoms, not the creation or destruction of matter.
Implications of the Law of Conservation of Mass
The implications of this law are profound and far-reaching:
- Predicting Reaction Outcomes: By balancing chemical equations, we can accurately predict the amounts of reactants needed and the amounts of products formed in a reaction. This is essential in industrial processes, chemical synthesis, and many other applications.
- Stoichiometry Calculations: Stoichiometry is the quantitative study of reactants and products in chemical reactions. Balancing equations is the foundation of stoichiometric calculations, allowing us to determine the ratios of reactants and products and perform calculations related to mass, moles, and volumes.
- Understanding Chemical Reactions: The law helps us understand the fundamental nature of chemical reactions. It highlights the fact that atoms are conserved during the process, merely rearranging themselves to form new molecules.
- Environmental Impact Assessment: In environmental chemistry, the law helps in assessing the environmental impact of chemical reactions. It allows us to track the fate of pollutants and predict their environmental distribution.
Beyond the Basic: Advanced Considerations
While the Law of Conservation of Mass perfectly represents balanced chemical equations in most cases, some nuances deserve attention:
Nuclear Reactions: An Exception
The Law of Conservation of Mass applies primarily to chemical reactions. In nuclear reactions, which involve changes in the atom's nucleus, a small amount of mass is converted into energy according to Einstein's famous equation, E=mc². In nuclear reactions, the total mass of reactants and products might not be exactly the same because some mass is converted to energy.
Open Systems: The Role of Mass Transfer
The Law of Conservation of Mass strictly holds true only for closed systems, where no matter enters or leaves. In open systems, where mass transfer occurs (e.g., gases escaping or being added), the total mass within the system might not remain constant. However, the law remains valid if we consider the mass entering and leaving the system.
Practical Applications and Examples
The principle of a balanced chemical equation representing the Law of Conservation of Mass finds widespread application in numerous fields:
Industrial Chemistry:
Manufacturing processes rely heavily on precise stoichiometric calculations derived from balanced chemical equations. This ensures efficient use of raw materials, minimizes waste, and optimizes product yield. Examples include the Haber-Bosch process for ammonia synthesis and the production of various polymers.
Environmental Science:
Assessing the impact of pollutants and modeling chemical reactions in the environment requires balanced equations to track the transformation and fate of pollutants. This is crucial for understanding atmospheric chemistry, water pollution, and soil contamination.
Biochemistry and Medicine:
Metabolic pathways in living organisms are complex sequences of chemical reactions. Balancing the equations representing these reactions is critical for understanding metabolic processes and developing new drugs and therapies.
Forensic Science:
In forensic analysis, identifying substances and determining the sequence of events in a crime scene often involve analyzing chemical reactions. Balanced equations are essential for accurate interpretations.
Conclusion: The Inseparable Link
The Law of Conservation of Mass and balanced chemical equations are inextricably linked. A balanced chemical equation is a visual and quantitative representation of this fundamental law. Mastering the art of balancing equations is not just a skill for chemistry students; it's a crucial tool for anyone working with chemical reactions in any field. Its importance stretches far beyond the classroom, influencing various scientific, industrial, and environmental applications. By understanding this fundamental relationship, we gain a deeper insight into the world of chemistry and its impact on our lives. Furthermore, the consistent application of this principle fosters accuracy, efficiency, and a deeper understanding of the intricate processes shaping our world.
Latest Posts
Latest Posts
-
Air Moves From High To Low Pressure
Apr 02, 2025
-
The Smallest Particle Of An Element Is A N
Apr 02, 2025
-
How Many Neutrons Does A Hydrogen Atom Have
Apr 02, 2025
-
What Is Nickel Used For In Everyday Life
Apr 02, 2025
-
A Tetrad Is Made Up Of
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Which Law Represents A Balanced Chemical Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
