A Substance That Cannot Be Broken Down By Chemical Means
Juapaving
Mar 31, 2025 · 6 min read
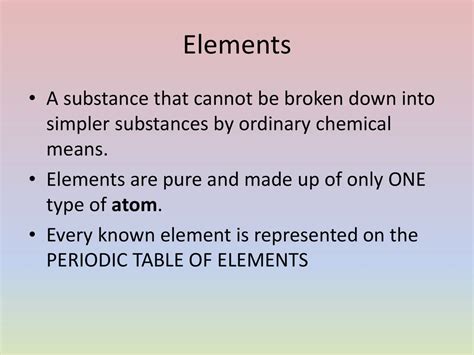
Table of Contents
The Unbreakable: Exploring the Nature of Elements
The quest to understand the fundamental building blocks of matter has captivated humanity for millennia. From ancient Greek philosophers pondering the nature of earth, air, fire, and water, to modern scientists utilizing particle accelerators, the drive to unravel the secrets of the universe persists. Central to this understanding is the concept of a substance that cannot be broken down by chemical means: an element. This article delves deep into the fascinating world of elements, exploring their properties, their discovery, their arrangement within the periodic table, and their crucial role in shaping the universe as we know it.
What Defines an Element?
At its core, an element is a pure substance consisting entirely of one type of atom. An atom, in turn, is defined by the number of protons in its nucleus—a quantity known as its atomic number. This atomic number uniquely identifies each element. For example, hydrogen (H), with one proton, is distinct from helium (He), with two protons, and lithium (Li), with three protons, and so on.
Crucially, elements cannot be broken down into simpler substances through chemical reactions. Chemical reactions involve the rearrangement of atoms, the breaking and forming of chemical bonds, but they do not alter the fundamental nature of the atoms themselves. While elements can participate in chemical reactions, forming molecules and compounds, they retain their atomic identity throughout the process. This fundamental property distinguishes elements from compounds and mixtures.
Distinguishing Elements from Compounds and Mixtures
It's essential to understand the difference between elements, compounds, and mixtures.
-
Elements: These are pure substances composed of only one type of atom. Examples include oxygen (O), iron (Fe), and gold (Au).
-
Compounds: These are substances formed when two or more different elements are chemically bonded together in fixed proportions. The properties of a compound are distinctly different from the properties of its constituent elements. Water (H₂O), for instance, is a compound formed from hydrogen and oxygen, exhibiting properties drastically different from its components.
-
Mixtures: These are combinations of two or more substances that are not chemically bonded. The components retain their individual properties, and the proportions can vary. Air, a mixture of gases including oxygen, nitrogen, and carbon dioxide, is a prime example. Unlike compounds, mixtures can be easily separated into their constituents by physical means like distillation or filtration.
The Periodic Table: A Systematic Organization of Elements
The periodic table, arguably one of the most significant achievements in science, is a tabular arrangement of the chemical elements, organized on the basis of their atomic number, electron configurations, and recurring chemical properties. Its structure reveals periodic trends—regular patterns in the properties of elements as their atomic numbers increase. These trends are a direct consequence of the arrangement of electrons in atoms and their influence on chemical behavior.
Understanding Periodic Trends
Several crucial periodic trends are observable:
-
Electronegativity: This refers to an atom's ability to attract electrons in a chemical bond. Electronegativity generally increases across a period (from left to right) and decreases down a group (from top to bottom).
-
Ionization Energy: This is the energy required to remove an electron from an atom. Ionization energy generally increases across a period and decreases down a group.
-
Atomic Radius: This measures the size of an atom. Atomic radius generally decreases across a period and increases down a group.
-
Metallic Character: This describes the tendency of an element to lose electrons and form positive ions. Metallic character generally decreases across a period and increases down a group.
These periodic trends are invaluable in predicting the chemical behavior of elements and their reactivity. They provide a framework for understanding the formation of compounds and the properties of materials.
The Discovery and Classification of Elements
The discovery of elements has been a gradual process, spanning centuries of scientific inquiry. Early alchemists, while operating with flawed theories, made significant contributions by isolating and characterizing various substances. The development of modern chemistry, with its emphasis on quantitative experimentation and the understanding of atomic structure, greatly accelerated the pace of element discovery.
Key Milestones in Element Discovery
-
Ancient Times: Several elements, such as gold (Au), silver (Ag), copper (Cu), iron (Fe), tin (Sn), lead (Pb), mercury (Hg), sulfur (S), and carbon (C), were known to ancient civilizations.
-
The 18th and 19th Centuries: The development of sophisticated laboratory techniques led to the discovery of numerous elements, notably oxygen, hydrogen, and many other elements prominent in the periodic table. Antoine Lavoisier's work in developing a system of chemical nomenclature was crucial in laying the groundwork for modern chemistry.
-
The 20th and 21st Centuries: The advancement of spectroscopic techniques and particle accelerators allowed the discovery and characterization of synthetic elements—elements not found in nature but created in laboratories. These synthetic elements often have very short half-lives, existing only for fractions of a second.
The Role of Elements in the Universe
Elements are not merely abstract concepts; they are the fundamental constituents of all matter in the universe. Their abundance and distribution vary dramatically, reflecting the processes of stellar nucleosynthesis—the creation of elements within stars.
Stellar Nucleosynthesis: The Cosmic Forge
Stars are colossal nuclear reactors, where lighter elements are fused together to form heavier elements. This process, known as stellar nucleosynthesis, is responsible for the creation of most elements heavier than hydrogen and helium. Different types of stars produce different elements, depending on their mass and lifespan. Supernova explosions, the dramatic deaths of massive stars, are particularly important in the synthesis and dispersal of heavy elements throughout the universe.
Elements and the Building Blocks of Life
The elements essential for life are relatively abundant in the universe but are specifically concentrated on Earth. These elements include:
-
Carbon (C): Forms the backbone of organic molecules.
-
Hydrogen (H): A key component of water and organic molecules.
-
Oxygen (O): Essential for respiration and a key component of water.
-
Nitrogen (N): A crucial part of amino acids and nucleic acids.
-
Phosphorus (P): A vital component of DNA and ATP.
-
Sulfur (S): Present in some amino acids.
The specific arrangement and interaction of these elements give rise to the incredible complexity and diversity of life on Earth.
Conclusion: An Ongoing Exploration
The study of elements remains a dynamic and evolving field. While we have made remarkable progress in understanding their properties, their arrangement, and their role in the universe, much remains to be discovered. The search for new elements, the investigation of their exotic properties, and the unraveling of the intricacies of their behavior continue to drive scientific research, pushing the boundaries of our understanding of the fundamental building blocks of reality. The elements, these seemingly simple entities, hold the key to comprehending the vastness and complexity of the universe, from the smallest atom to the largest star. The unbreakable nature of elements at a chemical level forms the solid foundation upon which all matter is built, a testament to the enduring power of fundamental scientific principles.
Latest Posts
Latest Posts
-
Air Moves From High To Low Pressure
Apr 02, 2025
-
The Smallest Particle Of An Element Is A N
Apr 02, 2025
-
How Many Neutrons Does A Hydrogen Atom Have
Apr 02, 2025
-
What Is Nickel Used For In Everyday Life
Apr 02, 2025
-
A Tetrad Is Made Up Of
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about A Substance That Cannot Be Broken Down By Chemical Means . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
