Hcn Is Weak Or Strong Acid
Juapaving
Apr 07, 2025 · 5 min read
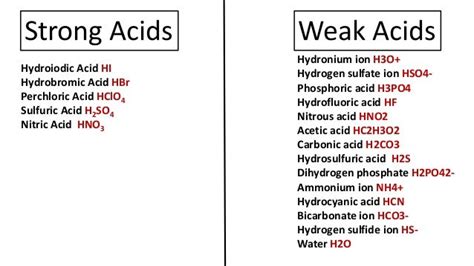
Table of Contents
Is HCN a Weak or Strong Acid? A Comprehensive Analysis
Hydrogen cyanide (HCN), also known as prussic acid, is a highly toxic chemical compound. But beyond its dangerous nature lies a fascinating chemical property: its acidity. The question of whether HCN is a weak or strong acid is crucial to understanding its behavior in various chemical reactions and its environmental impact. This article delves into the intricacies of HCN's acidity, exploring its dissociation constant, comparing it to other acids, and discussing the implications of its classification.
Understanding Acid Strength: The Ka Value
The strength of an acid is fundamentally determined by its ability to donate a proton (H⁺) in aqueous solution. Strong acids completely dissociate into their ions, while weak acids only partially dissociate. This dissociation is quantified by the acid dissociation constant, Ka. A higher Ka value indicates a stronger acid.
The dissociation of HCN in water is represented by the following equilibrium reaction:
HCN(aq) + H₂O(l) ⇌ H₃O⁺(aq) + CN⁻(aq)
The Ka expression for this reaction is:
Ka = [H₃O⁺][CN⁻] / [HCN]
The Ka value for HCN is approximately 6.2 x 10⁻¹⁰. This relatively small value is the key to understanding why HCN is classified as a weak acid.
Comparing Ka Values: HCN vs. Strong Acids
To put HCN's Ka value into perspective, let's compare it to some strong acids:
- Hydrochloric acid (HCl): Ka is very large (essentially complete dissociation)
- Sulfuric acid (H₂SO₄): Ka₁ is very large, Ka₂ is moderate.
- Nitric acid (HNO₃): Ka is very large.
The dramatic difference in Ka values clearly highlights the significant difference in acid strength between HCN and these strong acids. Strong acids readily donate their protons, leading to high concentrations of H₃O⁺ ions in solution. HCN, on the other hand, only partially dissociates, resulting in a much lower concentration of H₃O⁺ ions.
The Implications of HCN's Weak Acidity
The weak acidity of HCN has several significant implications:
1. Limited Dissociation and Low H₃O⁺ Concentration:
As a weak acid, HCN only partially dissociates in water. This means that the concentration of hydronium ions (H₃O⁺), which determine the acidity of the solution, remains relatively low. This is a crucial factor when considering the pH of HCN solutions and their reactivity.
2. Equilibrium Considerations:
The equilibrium nature of HCN's dissociation is crucial. The reaction doesn't proceed to completion; rather, a dynamic equilibrium exists between undissociated HCN and its ions (H₃O⁺ and CN⁻). This equilibrium can be shifted by changing conditions such as the concentration of reactants or products, or by adjusting the temperature. Understanding this equilibrium is vital in predicting the behavior of HCN in different environments.
3. Reaction Rates and Selectivity:
HCN's weak acidity affects its reaction rates. Since only a small fraction of HCN molecules dissociate, the concentration of reactive species (H₃O⁺) is limited. This can lead to slower reaction rates compared to reactions involving strong acids. Furthermore, the weak acidity can influence the selectivity of reactions, favoring certain pathways over others.
4. Environmental Behavior:
The weak acidity of HCN influences its behavior in the environment. For instance, its low dissociation means it doesn't readily contribute to the acidity of natural water bodies. However, its toxicity remains a major concern, regardless of its weak acidity. The cyanide ion (CN⁻), even though it's the conjugate base of a weak acid, is highly toxic and poses significant environmental and health risks.
Factors Influencing HCN's Acidity
While the Ka value provides a quantitative measure of HCN's acidity, other factors also influence its behavior:
1. Temperature:
Temperature affects the equilibrium of the dissociation reaction. Increasing the temperature generally increases the dissociation of weak acids, leading to a slightly higher Ka value and a more acidic solution. However, the effect is usually not dramatic for weak acids like HCN.
2. Solvent Effects:
The solvent in which HCN is dissolved significantly impacts its acidity. Water is the typical solvent considered, but other solvents with different dielectric constants and hydrogen bonding capabilities can alter the extent of dissociation and consequently the effective acidity.
3. Concentration:
The concentration of HCN also plays a role. While the Ka value remains constant at a given temperature, the actual concentration of H₃O⁺ ions will increase with increasing HCN concentration. However, even at high concentrations, HCN remains a weak acid due to its inherently low dissociation tendency.
HCN's Toxicity: A Separate Concern
It's critical to emphasize that the weak acidity of HCN does not diminish its extreme toxicity. HCN's toxicity is primarily due to its ability to inhibit cytochrome c oxidase, a crucial enzyme in cellular respiration. This inhibition leads to cellular hypoxia and ultimately death. The toxic effects of HCN are independent of its weak acidic nature and are a separate concern that requires stringent safety precautions.
Conclusion: HCN - A Weak Acid with Serious Implications
In conclusion, hydrogen cyanide (HCN) is definitively classified as a weak acid based on its low Ka value (approximately 6.2 x 10⁻¹⁰). This weak acidity significantly affects its behavior in various chemical reactions and its environmental impact. Its partial dissociation leads to low concentrations of hydronium ions, influencing reaction rates and equilibrium considerations. However, it's paramount to remember that the weak acidity of HCN does not mitigate its extreme toxicity. The lethal effects of HCN are a serious concern that must be addressed through proper handling and safety protocols. Understanding both the weak acidity and the potent toxicity of HCN is crucial for anyone working with this compound or studying its properties. Further research continues to refine our understanding of HCN's complex chemical behavior and its impact on various systems.
Latest Posts
Latest Posts
-
Lcm Of 3 6 And 7
Apr 08, 2025
-
The Three Medians Of A Triangle Intersect At The
Apr 08, 2025
-
What Is The Difference Between Cell Wall And Cell Membrane
Apr 08, 2025
-
How Many Hours In A Leap Year
Apr 08, 2025
-
What Is The Lcm Of 6 12 And 15
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Hcn Is Weak Or Strong Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.