Explain The Basis For The Great Diversity Of Proteins
Juapaving
Apr 04, 2025 · 6 min read
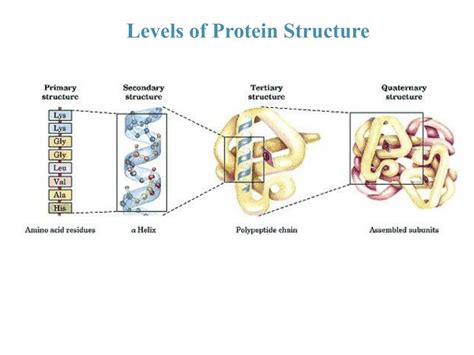
Table of Contents
Explain the Basis for the Great Diversity of Proteins
Proteins are the workhorses of the cell, carrying out a vast array of functions crucial for life. Their incredible diversity underpins the complexity of living organisms, from the simplest bacteria to the most sophisticated mammals. But how is this immense variety generated from a seemingly limited set of building blocks? The answer lies in a complex interplay of factors, including the genetic code, post-translational modifications, and the intricate three-dimensional structures proteins adopt.
The Genetic Code: The Foundation of Protein Diversity
The primary basis for protein diversity lies within the genetic code itself. This code, encoded in DNA and transcribed into messenger RNA (mRNA), dictates the sequence of amino acids that make up a protein. There are 20 standard amino acids, each with unique chemical properties – some are hydrophobic (water-repelling), others hydrophilic (water-attracting), some are charged, and others are uncharged. The precise arrangement of these amino acids in a polypeptide chain is determined by the sequence of nucleotide triplets (codons) in the mRNA.
The Role of Codons and Amino Acid Sequence
Each codon specifies a particular amino acid. Since there are four nucleotides (adenine, guanine, cytosine, and uracil) in mRNA, and each codon consists of three nucleotides, there are 4³ = 64 possible codons. However, only 20 amino acids are used in protein synthesis. This redundancy, where multiple codons can code for the same amino acid, is a feature of the genetic code. This redundancy allows for some mutations in the DNA sequence to occur without altering the amino acid sequence of the resulting protein, a crucial aspect of genetic robustness.
The Impact of Gene Mutations
Variations in the DNA sequence, known as mutations, can lead to changes in the amino acid sequence of a protein. These changes can be subtle, affecting only a single amino acid, or they can be more drastic, involving insertions, deletions, or larger-scale rearrangements of genetic material. Such mutations are a major driver of protein diversity, leading to the creation of novel protein variants with potentially altered functions.
- Point mutations: These are single nucleotide changes that can lead to either a silent mutation (no change in amino acid sequence), a missense mutation (change in a single amino acid), or a nonsense mutation (premature termination of protein synthesis).
- Insertions and deletions: These additions or removals of nucleotides can cause frameshift mutations, dramatically altering the amino acid sequence downstream of the mutation.
- Gene duplication and divergence: Duplication of an entire gene followed by mutations in the duplicated copy can lead to the evolution of new protein functions. This is a major mechanism for generating new proteins with novel properties.
Post-Translational Modifications: Adding Complexity to Protein Diversity
The diversity of proteins is further amplified by a wide range of post-translational modifications (PTMs). These are chemical alterations that occur after a protein is synthesized, often impacting its function, localization, or stability. PTMs can occur on specific amino acid residues, affecting their properties and influencing the overall protein structure and activity.
Common Types of Post-Translational Modifications
Numerous types of PTMs exist, including:
- Phosphorylation: The addition of a phosphate group to serine, threonine, or tyrosine residues. This is a common regulatory mechanism, often switching protein activity on or off.
- Glycosylation: The addition of carbohydrate groups, which can affect protein folding, stability, and cell signaling.
- Ubiquitination: The attachment of ubiquitin molecules, often targeting proteins for degradation.
- Acetylation: The addition of an acetyl group, commonly affecting histone proteins and regulating gene expression.
- Methylation: The addition of a methyl group, which can alter protein-protein interactions or enzymatic activity.
- Lipidation: The attachment of lipid molecules, anchoring proteins to cell membranes.
The combinatorial nature of PTMs significantly expands the functional diversity of proteins. A single protein can undergo multiple PTMs simultaneously, resulting in a wide range of modified forms with distinct properties. This allows for precise regulation of protein function in response to various cellular signals and environmental cues.
Protein Folding and Three-Dimensional Structure: The Key to Function
The linear amino acid sequence of a protein dictates its three-dimensional structure. This structure, encompassing primary, secondary, tertiary, and quaternary levels, is crucial for protein function. The diverse folding patterns, driven by interactions between amino acid side chains, give rise to a vast array of protein shapes and functionalities.
Levels of Protein Structure
- Primary structure: The linear sequence of amino acids.
- Secondary structure: Local folding patterns, such as alpha-helices and beta-sheets, stabilized by hydrogen bonds.
- Tertiary structure: The overall three-dimensional arrangement of a polypeptide chain, stabilized by various interactions, including hydrophobic interactions, hydrogen bonds, ionic bonds, and disulfide bridges.
- Quaternary structure: The arrangement of multiple polypeptide chains (subunits) to form a functional protein complex.
The Impact of Protein Structure on Function
The precise three-dimensional structure of a protein determines its function. Small changes in the amino acid sequence can drastically alter the protein's folding pattern, potentially leading to loss of function or the acquisition of new functions. The intricate folding processes are often assisted by chaperone proteins, ensuring proper folding and preventing the formation of misfolded, potentially harmful aggregates.
The diverse range of protein folds enables proteins to interact with a wide variety of molecules, performing diverse functions such as catalysis, transport, structural support, signaling, and regulation.
Protein Domains and Modules: Building Blocks of Complexity
Many proteins are composed of modular units called domains or modules. These are independently folding regions within a protein that often have specific functions. The combination of different domains within a single protein generates a vast repertoire of functional possibilities.
Domain Shuffling and Evolution
Evolutionary processes, such as exon shuffling and gene duplication, can lead to the creation of new proteins through the combination of existing domains. This “domain shuffling” is a significant driver of protein diversity, enabling the creation of complex proteins with novel functionalities. This mechanism allows for the rapid evolution of proteins by combining pre-existing functional units, rather than requiring the evolution of entirely new protein structures from scratch.
Conclusion: A Symphony of Mechanisms
The immense diversity of proteins arises from a sophisticated interplay of mechanisms. The genetic code provides the fundamental blueprint, specifying the amino acid sequence. Post-translational modifications add layers of complexity, further diversifying protein function. The intricate three-dimensional structures proteins adopt, driven by amino acid interactions and assisted by chaperones, determine their specific functions. Finally, modular domains and their combinatorial arrangements allow for the rapid evolution of new proteins with complex functionalities. This intricate interplay results in the astounding variety of proteins that underpin the incredible complexity of life. The continuing study of these mechanisms promises to unveil further insights into the remarkable diversity and functionality of the protein world.
Latest Posts
Latest Posts
-
Five Letter Words Starting With Ri
Apr 05, 2025
-
How To Find Factors Of Equation
Apr 05, 2025
-
Does A Rhombus Have Parallel Lines
Apr 05, 2025
-
Descriptive Words That Start With S
Apr 05, 2025
-
50 Is What Percent Of 500
Apr 05, 2025
Related Post
Thank you for visiting our website which covers about Explain The Basis For The Great Diversity Of Proteins . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
