Elements And Compounds Are Two Types Of
Juapaving
Mar 25, 2025 · 6 min read
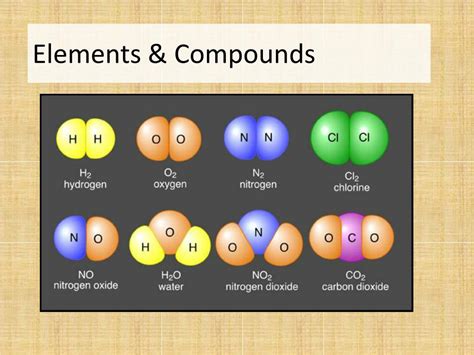
Table of Contents
Elements and Compounds: Two Fundamental Types of Matter
Chemistry, at its core, is the study of matter and its transformations. Matter, anything that occupies space and has mass, exists in countless forms, from the air we breathe to the intricate structures within our bodies. Understanding the fundamental building blocks of matter is crucial to comprehending the world around us. These building blocks are primarily categorized into two distinct types: elements and compounds. This article will delve deep into the characteristics, differences, and examples of elements and compounds, providing a comprehensive understanding of their roles in the vast landscape of chemistry.
What are Elements?
Elements are the simplest form of pure substances. They are made up of only one type of atom, meaning all the atoms within an element have the same number of protons in their nucleus. This number of protons, known as the atomic number, uniquely identifies each element. Elements cannot be broken down into simpler substances by any chemical means. While they can undergo physical changes, such as melting or boiling, their fundamental atomic structure remains intact.
Key Characteristics of Elements:
- Pure Substances: Elements consist entirely of one type of atom. There are no other elements or compounds mixed in.
- Unique Atomic Number: Each element is defined by its unique atomic number, representing the number of protons in the nucleus of its atoms.
- Indivisible by Chemical Means: Elements cannot be broken down into simpler substances through chemical reactions. Nuclear reactions, however, can transform elements.
- Periodic Table Organization: All known elements are organized and categorized in the periodic table, a crucial tool in chemistry. The periodic table arranges elements based on their atomic number and recurring chemical properties.
- Diverse Physical and Chemical Properties: Elements exhibit a vast range of physical properties, including density, melting point, boiling point, and conductivity. Their chemical properties, such as reactivity and bonding behavior, also vary greatly.
Examples of Elements:
The periodic table lists over 118 elements, ranging from the simplest, hydrogen (H), to the complex, such as uranium (U). Some commonly known elements include:
- Hydrogen (H): The lightest and most abundant element in the universe.
- Oxygen (O): Essential for respiration and combustion.
- Carbon (C): The foundation of organic chemistry, forming the basis of all living things.
- Nitrogen (N): A major component of the Earth's atmosphere and crucial for plant growth.
- Iron (Fe): A strong and versatile metal used in numerous applications.
- Gold (Au): A precious metal known for its inertness and malleability.
- Chlorine (Cl): A highly reactive halogen used in water purification.
What are Compounds?
Unlike elements, compounds are pure substances composed of two or more different elements chemically bonded together in fixed proportions. This chemical bonding creates a new substance with properties distinct from its constituent elements. Compounds can be broken down into their constituent elements through chemical reactions, such as electrolysis or decomposition.
Key Characteristics of Compounds:
- Chemical Bonding: Atoms within a compound are held together by strong chemical bonds, such as ionic or covalent bonds.
- Fixed Proportions: The elements in a compound always combine in the same fixed ratio by mass. This is known as the Law of Definite Proportions.
- Distinct Properties: Compounds possess properties that are different from the properties of the elements that make them up. For example, sodium (a highly reactive metal) and chlorine (a toxic gas) combine to form sodium chloride (table salt), a stable and edible compound.
- Decomposable by Chemical Means: Compounds can be broken down into their constituent elements through chemical reactions.
- Chemical Formulas: Compounds are represented by chemical formulas, which indicate the types and numbers of atoms present in each molecule or formula unit.
Types of Chemical Bonds in Compounds:
Two primary types of chemical bonds form compounds:
- Ionic Bonds: These bonds are formed by the electrostatic attraction between oppositely charged ions. One atom loses electrons (becoming a positive ion or cation) and another atom gains those electrons (becoming a negative ion or anion). Example: Sodium chloride (NaCl).
- Covalent Bonds: These bonds are formed by the sharing of electrons between atoms. This sharing results in a stable molecule. Example: Water (H₂O).
Examples of Compounds:
The world is filled with countless compounds, each with its unique properties and applications. Some common examples include:
- Water (H₂O): Essential for life and a crucial solvent.
- Carbon Dioxide (CO₂): A greenhouse gas and product of respiration.
- Sodium Chloride (NaCl): Table salt, crucial for maintaining electrolyte balance in the body.
- Glucose (C₆H₁₂O₆): A simple sugar, a primary source of energy for living organisms.
- Table Sugar (Sucrose, C₁₂H₂₂O₁₁): A disaccharide composed of glucose and fructose.
- Ethanol (C₂H₅OH): A type of alcohol, used in alcoholic beverages and as a solvent.
- Ammonia (NH₃): Used in fertilizers and cleaning products.
Distinguishing Between Elements and Compounds
The key differences between elements and compounds are summarized below:
| Feature | Element | Compound |
|---|---|---|
| Composition | One type of atom | Two or more different types of atoms |
| Bonding | No chemical bonds between atoms | Chemical bonds (ionic or covalent) present |
| Decomposition | Cannot be broken down chemically | Can be broken down chemically into elements |
| Properties | Properties unique to the element | Properties different from constituent elements |
| Representation | Symbol (e.g., H, O, Fe) | Chemical formula (e.g., H₂O, NaCl, CO₂) |
Mixtures: A Third Category of Matter
While elements and compounds are pure substances, it's important to also consider mixtures. Mixtures are combinations of two or more substances (elements, compounds, or both) that are not chemically bonded. The components of a mixture retain their individual properties and can be separated by physical means, such as filtration, distillation, or evaporation.
Examples of mixtures include:
- Air: A mixture of gases, primarily nitrogen and oxygen.
- Seawater: A mixture of water and various salts and minerals.
- Sand: A mixture of different mineral particles.
- Soil: A complex mixture of minerals, organic matter, and water.
The Importance of Elements and Compounds
Understanding the fundamental differences between elements and compounds is crucial in various scientific fields. In medicine, for instance, knowledge of the chemical composition of drugs and their interactions with the body is vital. In materials science, the properties of different elements and compounds dictate the characteristics of materials used in construction, electronics, and many other applications. In environmental science, understanding the chemical cycles of elements and compounds helps us address environmental issues like pollution and climate change.
Conclusion
Elements and compounds represent the foundational building blocks of matter. Elements, the simplest form of pure substances, consist of only one type of atom. Compounds, on the other hand, are formed by the chemical combination of two or more different elements in fixed proportions, resulting in substances with unique properties. By understanding the distinctions and characteristics of elements and compounds, we gain a deeper appreciation for the complexity and beauty of the chemical world and its impact on our lives. This knowledge is crucial for advancements in various scientific fields and for tackling some of the world's most pressing challenges. Further exploration into the periodic table and the intricacies of chemical bonding will reveal even more fascinating aspects of this fundamental concept in chemistry.
Latest Posts
Latest Posts
-
Moment Of Inertia Of Quarter Circle
Mar 28, 2025
-
What Are Common Multiples Of 6 And 8
Mar 28, 2025
-
State Newtons Law Of Universal Gravitation In Words
Mar 28, 2025
-
Is 6 A Factor Of 84
Mar 28, 2025
-
What Is The Lcm Of 2 And 11
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Elements And Compounds Are Two Types Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
